Abstract
Objective An in-vitro study to examine the correlation between the distribution of the autofluorescent signal emitted from carious dentine (detected using confocal laser scanning microscopy) and its microhardness, within the depths of human dentine lesions.
Materials and methods Twelve carious teeth were sectioned longitudinally, the cut faces marked with a grid reference line system and colour photomicrographs taken. The same samples were imaged using confocal laser-scanning microscopy for autofluorescence and then subjected to microhardness testing using a Knoop microhardness indenter. Adjacent sound dentine was used as a control reference. Digital image superimposition allowed direct comparisons to be made between the colour, autofluorescence and microhardness of each lesion.
Results Sound enamel and dentine did not autofluoresce. Autofluorescence distribution from carious dentine correlated with the highly softened tissue (detected using the Knoop indenter) and terminated at a level superficial to the translucent zone. This zone was still pigmented. Normal, sound dentine hardness levels were found deep to the translucent zone.
Conclusions A correlation existed between the zone of autofluorescence and carious dentine that was markedly softened by the carious process. These findings highlighted a possibility that the autofluorescence might be used as an in-vitro, objective histological marker for the softened, carious dentine requiring clinical excavation.
Similar content being viewed by others
Main
Currently, the physical parameter used most commonly by dental practitioners to guide clinical excavation of infected, carious dentine is the hardness/texture of the carious tissue and, less importantly, the colour of the affected dentine1,2 as well as caries detector dyes.3 All of these methods suffer from the subjectivity inherent between dentists in clinical practice, which is likely to result in variations in the quality and quantity of dentine removed during cavity preparation. These variations may have serious clinical implications such as differences in the size of the cavities produced, the pulpal health beneath prepared cavities and the strength of the remaining tooth structure. In addition, the quality of the dentine remaining at the base of a cavity may have an effect on the bonding of some adhesive restorative materials.4
Hardness has been associated with the relative infectivity of carious dentine, helping the dentist distinguish between either heavily infected (soft) or minimally infected (hard) dentine.1,2 However, it is a clinically subjective parameter that will be modified by a variety of other factors that should be considered. For instance, the changing orientation and density of the tubules from the enamel-dentine junction (EDJ) to the pulpal interface may play an important part in the differences in hardness found between these areas in healthy dentine.5 Atomic force microscopy (AFM) has been used to measure the hardness of a fully hydrated, sound dentine sample6 and this nanoindentation method has produced results indicating that the differences in dentine hardness relative to its location might actually be attributed to changes in the hardness of hydrated intertubular dentine and not necessarily to an increase in the number of tubules alone.
The hardness of dentine also depends upon the state of mineralisation of the tissue. Dentine mineral is composed of magnesium- and carbonate-substituted calcium hydroxyapatite crystals organised in a crystal lattice; this lattice structure affords the hardness of the healthy tissue. Mantle dentine (subjacent to the EDJ) has been shown to be relatively hypomineralised with poorer fusion of calco-spherites7 and has a greater network of terminally branching tubules;8 these might be reasons for greater porosity and subsequent reduction in hardness measurements that have been shown experimentally.9,10 In carious dentine, the hardness will also depend on the age of the lesion and its stage of progression; advanced, active lesions tend to be soft while 'arrested' lesions tend to become harder with time, probably caused by the remineralisation process.11,12,13 The hypermineralised dentine deep to the advancing edge of a carious lesion (the translucent zone) could be caused by the liberation of the calcium and phosphate from the body of the lesion with subsequent physico-chemical precipitation in this region.14 Because of the differences in crystal structure, these newly formed plate-like crystals and rhomboidal, magnesium-substituted tricalcium phosphate complexes (whitlockite) will not afford the same hardness values as the lattice structure of normal hydroxyapatite.15
Early studies of the hardness of sound dentine used a microhardness tester with a Knoop diamond indenter to determine the hardness values of coronal dentine.9,16 Not surprisingly, it was noted that carious dentine was softer and the peripheral dentine surrounding the carious lesion was harder than sound dentine at equivalent depths. Fusayama et al. attempted to find a relationship between hardness, discoloration and bacterial invasion in carious dentine.17 They concurred with the previous findings that dentine close to the pulp was considerably softer than the more superficial, circumpulpal dentine and realised that, in the depths of the lesion, softening preceded discoloration which in turn occurred before bacterial invasion. Differences were also found between acute and chronic lesions, implying that advancing fronts of acute lesions were significantly softer than their chronic counterparts.18
In the light of the above findings, it seems sensible, therefore, to attempt to develop an objective marker for excavatable carious dentine. This objectivity is needed both in the research laboratory as well as the clinic. These are interesting times with new techniques including air abrasion19 and Carisolv™ gel20 becoming available to practitioners.21 These techniques require laboratory and clinical evaluation but at present there are no clear, objective histological markers available to delineate that infected dentine which ought to be removed. The use of natural fluorescence (or autofluorescence (AF)) to delineate the carious dentine requiring excavation is currently under investigation. In-vitro studies have shown that carious dentine exhibits natural autofluorescence (AF) detectable using confocal laser scanning microscopy (CLSM).22,23 This methodology allowed the AF signal to be accurately related to the overall structure of the carious lesion in the laboratory. In studies mimicking clinical caries removal in vitro, a correlation was found between the amount of carious dentine excavated using a hand excavator and the distribution of the AF signal.23 In this instance, tissue excavation was guided by the hardness of dentine; excavation was terminated at the level where hard dentine was first detected using a dental probe. Results implied that the AF was associated with the relative hardness of carious dentine as determined by a dentist during excavation.23 This hypothesis is to be investigated further in the following experiment which looks to see if there is a correlation between the autofluorescent signal and the microhardness of dentine.
Materials and method
Sample preparation
Samples were prepared from 12 carious, permanent, human teeth that had been stored whole in de-ionised water at 4°C for a maximum of 2 weeks post extraction from fit and healthy adults. Teeth were examined under a dissecting microscope and excluded from the investigation if there was any evidence of cracks, flaws or extraction damage. All the samples used were clinically assessed for occlusal caries using good illumination and magnification after they had been cleaned with a toothbrush and then washed and dried using a three-in-one syringe. Cavitated teeth were sectioned through the lesions in the mesio-distal longitudinal plane using a water-cooled diamond-impregnated circular saw (Labcut 1010, Agar Scientific Ltd, Stansted, Essex, UK) rotating at 100 rpm. The freshly cut surfaces were assessed with regards to lesion depth. If there was no visual evidence of a translucent zone about 1.5 mm from the EDJ (ie the lesions had penetrated into the final third of dentine toward the pulp) the samples were discarded from the study. To minimise errors caused by tilting and to avoid the introduction of stresses during the microhardness testing, each section was mounted and supported on a glass slide using green-stick composition and a paralleling device. The samples were kept hydrated at all times during the experiments. A grid reference pattern was scored onto the cut longitudinal surfaces using a scalpel blade. This would allow subsequent accurate superimposition of the images and data collected with the different modalities.
Microscopy
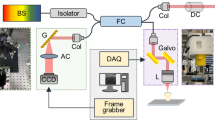
The prepared samples were then individually imaged using a Noran Odyssey CLSM (Middleton, WI, USA) in conjunction with a Leitz 2.5/0.08 dry objective. The argon ion laser excitation wavelength was 488 nm and the 515 nm barrier filter was used to detect the autofluorescence. A 15 μm confocal detection slit was selected, and standard gain and contrast settings were used throughout the experiment in order to prevent AF signal variations between individual samples. The resulting grayscale 512 × 512 × 8 bit images were captured using Metamorph image analysis software (v 1.1, Universal Imaging Corporation, PA, USA) and stored as '.tiff' files on PC hard disk. Reflected light photomicrographs were also captured on colour transparency film (Kodak Elite Ektachrome, ASA 400) using the same lens on an epi-illuminated light microscope. Using this protocol, all the images were subsequently digitised and alignment of the grid reference lines allowed direct superimposition of the confocal fluorescence and reflected light images to be accomplished.
Hardness
A Miniload 2 microhardness indenter (Leitz Wetzlar, Germany) fitted with a Knoop indenter (fig. 1) was used to generate diamond-shaped indentations within the specimens. These were mounted on the stage of the hardness tester so that there was no perceptible tilt or rock and orientated so that the long diagonal of each indentation was parallel to the EDJ. The surface of each sample was indented in rows tracking from the EDJ to the pulp in increments of 500 μm. In the carious samples, it was not always possible to start the measurements directly adjacent to the EDJ as the dentine in this region was grossly affected and so indentations were not clearly visible. In these instances, the measurements were taken within 100 μm of the EDJ. Both sound and carious dentine were indented; in total, there were more carious data points recorded than sound. It was found that, in sound dentine, a load of 0.98 N was required to produce indentations with long diagonals (fig. 1) of a size suitable for accurate measurement, while minimising surface damage. For carious dentine the variation of hardness throughout the lesion required a range of loads from 0.147 N superficially, to 0.98 N towards the advancing front, to be used to produce measurable long diagonals. Measurements of the length of the long diagonal of each indentation were estimated to 0.1 μm, the resolution of the fine scale of the instrument being 0.5 μm. Values of the Knoop hardness of sound and carious dentine were calculated according to the equation:

Where KHN = Knoop Hardness Number; F = force (N); L = long diagonal length (μm).
Results
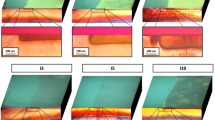
Figures 2a and 3a showed the KHN values superimposed upon the reflected light photomicrographs of two of the lesions from which the measurements were taken. Added to these were the corresponding autofluorescent signatures of the specific lesions (figs 2b and 3b); superimposition using the grid reference lines enabled visual comparisons to be made between the hardness, colour and AF of the dentine lesion. Each photomicrograph clearly showed the colour gradations found through carious dentine, with the darkly pigmented tissue just subjacent to the EDJ. From here, the dark brown pigmentation faded to brown, then to pale brown and finally into a glassy, opaque zone — the translucent zone. This translucent zone circumscribed the entire dentine lesion but was wider at the depths of the advancing front (figs 2a and 3a). Deep to this layer, the appearance of the dentine conformed to that which is accepted as being sound and unaffected by the carious process.
Colour photomicrograph showing an occlusal lesion in dentine with dark brown dentine just subjacent to the EDJ, then brown, pale brown and translucent (TD) towards the pulp (bottom of image; GRL — grid reference lines). Superimposed are KHN values (red dot : < 40 KHN; yellow dot: 40–49 KHN; green dot: 50–55 KHN; blue dot: > 55 KHN. Bar = 500 μm)
Colour photomicrograph of an approximal, cavitated dentine lesion again showing the pigment gradations through the lesion from EDJ to the pulp (TD — translucent dentine; GRL — grid reference lines). Again the KHN values have been superimposed (Red dot : < 40 KHN; Yellow dot: 40–49 KHN; Green dot: 50–55 KHN; Blue dot: > 55 KHN. Bar = 500 μm)
The hardness values were colour coded to indicate the microhardness range within each zone in the carious lesion as well as the sound adjacent dentine. From figures 2a, 3a and Table 1 it can be seen that sound circumpulpal dentine had a mean KHN value exceeding 55 (blue dots in figs 2 and 3) with a wide range between 55.77–64.17 KHN. Peripheral dentine, subjacent to the EDJ, recorded values between 48.88 and 53.91 KHN (yellow/green dots in figs 2 and 3). When examining carious dentine, the mean KHN values ranged between 13.64 and 37.93 (red dots in figs. 2 and 3) up to 1,000 μm from the EDJ. This zone incorporated the dark brown, brown and pale brown carious dentine zones visible in figures 2a and 3a. These zones and their corresponding depths in the lesions are highlighted in Table 1. Within the translucent zone (between 1,500 to 2,000 μm in the samples), the hardness values gradually increased to a peak towards its leading edge of 55.02 KHN; this value was within the range of KHN values documented for sound dentine at the equivalent depth (53.27–61.94 KHN). Sound dentine KHN values were also observed in zones deep to the translucent layer, towards the pulp.
On superimposing the corresponding AF signatures, the area of the AF signal followed the shape of the pigmented dentine lesion and the boundaries correlated closely with that dentine softened markedly by the carious process (red dots in figs 2 and 3). This correlation was found in all the samples tested. The AF did not emanate from the surrounding sound dental tissue or from the deeper translucent zone. The AF signal intensity peaked at the heart of the dentine lesion subjacent to the EDJ (figs 2b and 3b) and gradually reduced towards the advancing front. The zone of AF termination coincided with dentine that was pigmented (the pale brown zone); this area was superficial to the histological translucent zone.
Discussion
The methodology followed in this study allowed direct superimposition of different forms of data (ie hardness, autofluorescence and also the colour) collected from the same, individual sample. This, in turn, permitted accurate visual analysis of the results which indicated a correlation between the AF signal, the hardness of the affected tissue and the pigmentation of carious dentine.
Indentation increments of 500 μm were used as a previous study had showed this would allow sufficient spacing, so preventing crack propagation or other indentation damage to affect adjacent long diagonal readings.10 These effects were more pronounced in the carious dentine. Because the carious dentine at the EDJ was, in most cases, so soft and damaged, readings could not be accurately registered. Therefore, it was decided to take the readings within 100 μm of the EDJ. Further stages of sample preparation including resin inclusion and polishing as described in other similar experiments10 were avoided to ensure the samples were kept in as natural a state as possible and to minimise preparation artefacts.
To allow some comparison to be made between the hardness values recorded for sound and carious dentine, an attempt was made to standardise the lesion depths by only permitting lesions of a certain depth into the study (those with a translucent zone evident 1.5 mm from the EDJ). Since natural lesions were used, it was impossible to standardise all the variables that would occur between samples, eg size, age, state of activity and exact site. Therefore, care was needed in interpreting the comparative numerical data between sound and carious dentine.
In figures 2 to 3 the hardness values were superimposed on the images of the original dentine lesions and their autofluorescent signatures. It was evident that the autofluorescent signal correlated well with the more pigmented dentine which was bounded by the transparent and sub-transparent layers that descriptively and structurally make up the translucent zone.15 It was observed that the transparent zone is not made up from the hardest dentine found in the crown of the tooth. This harder sound tissue is found just subjacent to the transparent zone. For this reason, the alternative term for the translucent zone, sclerotic dentine, should be avoided as this is actually a misnomer – sclerosis means excessive hardening of tissues. These findings concur with those described by Ogawa et al. who argued that the transparent and sub-transparent divisions of the translucent zone of a dentine lesion are actually the deepest part of the lesion rather than a sound, protective, barrier zone created as a response to the advancing caries process by the pulp-dentine complex.15
The fact that hardness and AF distribution do not show an absolute correlation (ie the AF signal terminates in a zone of dentine that is still softened by the carious process) might be explained by the fact that generation of the AF signal requires a bacterial input, possibly by direct or indirect interactions with the dentine matrix.23 As it has been shown that there is no direct correlation between the AF distribution and the mineral content within dentine lesions,24 one would not necessarily expect to detect the AF signal in the dentine at the translucent advancing front of the lesion. At this depth, even though the acidic environment will have induced inorganic, structural changes at the crystal level affecting the hardness of the dentine (ie the production of plate-like crystals and whitlockite), bacterial proteolytic interactions will not yet have occurred within the matrix. This would therefore result in a lack of AF signal at this level. More superficially, where bacterially-induced matrix alteration/degradation will have occurred, the generation of the AF signal becomes apparent and detectable.
The results from this experiment seem to concur with those from the previous studies which have found that the sound dentine subjacent to the EDJ was relatively softer than that in the body of the tooth.9,15 This superficial dentine has a greater density of tubule branches and, with the possible altered crystallinity that has been proposed to occur in this layer, these factors might explain the observed reduced KHN values. As the pulpal surface is approached, the dentine becomes softer. Again, this is probably in part caused by the increased concentration of tubules in this area.25
Conclusions
-
Sound dentine adjacent to the EDJ and the pulp chamber was softer than the remaining sound, coronal dentine
-
The softest, carious dentine corresponded with the outer, brown-pigmented dentine and gradually the hardness increased to a peak just subjacent to the translucent zone (in the pulpal direction)
-
There is an association between the AF signal distribution and the microhardness of carious dentine. The AF signal distribution correlated with the softest dentine associated with the lesion and ended before the point at which the dentine hardness reached its normal value, ie a level superficial to the translucent zone (transparent layer), which was still pigmented (pale brown).
Clinical relevance
The AF signal terminates at a level at which clinical excavation of soft, carious dentine might reasonably be expected to terminate also.2 This histologically corresponds to the outer, irreversibly damaged carious zone.26 The deeper, translucent zone of dentine is currently accepted as representing the affected, but minimally infected, tissue which could be retained at the end of clinical cavity preparation. Adequately sealed restorations with bacteriocidal materials should arrest the carious process at this level. Therefore, the findings from this experiment provided evidence to support the use of the AF signature of a lesion as a more reproducible, objective, histological marker for the carious dentine requiring excavation in a dentine lesion.
Funding for this study was kindly provided by the MRC in the form of a Clinical Training Fellowship (grant no: G84/4339).
References
Kidd E A M, Joyston-Bechal S, Beighton D . Microbiological evaluation of caries activity during cavity preparatio. Caries Res 1993; 27: 402–408.
Kidd E A M, Ricketts D N J, Beighton D . Criteria for caries removal at the enamel-dentine junction: a clinical and microbiological study. Br Dent J 1996; 180: 287–291.
van de Rijke J W . Use of dyes in cariology. Int Dent J 1991; 41: 111–116.
Nakajima M, Sano H, Burrow M F et al. Tensile bond strength and SEM evaluation of caries-affected dentin using dentin adhesives. J Dent Res 1995; 74: 1679–1688.
Marshall G W Jr, Marshall S J, Kinney J H, Balooch M . The dentine substrate; structure and properties related to bonding. J Dent 1997; 25: 441–458.
Kinney J H, Balooch M, Marshall S J, Marshall G W Jr, Weihs T P . Hardness and Young's Modulus of human peritubular and intertubular dentine. Archs Oral Biol 1996; 41: 9–13.
Jones S J, Boyde A . Ultrastructure of dentin and dentinogenesis. Linde A, ed. Dentin and dentinogenesis Vol I. Florida: CRC Press Inc, 1984; 81–134.
Mjör I A, Nordahl I. The density and branching of dentinal tubules in human teeth. Archs Oral Biol 1996; 41: 401–412.
Craig R G, Gehring P E, Peyton F A . Relation of structure to the microhardness of human dentin. J Dent Res 1959; 38: 624–630.
Meredith N, Sherriff M, Setchell D J, Swanson S A V . Measurement of the microhardness and Young's Modulus of human enamel and dentine using an indentation technique. Archs Oral Biol 1996; 41: 539–545.
Miller W A, Massler M . Permeability and staining of active and arrested lesions in dentine. Br Dent J 1962; 112: 187–197.
Nyvad B, Fejerskov O. Active root surface caries converted into inactive caries as a response to oral hygiene. Scand J Dent Res 1986; 94: 281–284.
Bjørndal L, Larsen T, Thylstrup A . A clinical and microbiological study of deep carious lesions during stepwise excavation using long treatment intervals. Caries Res 1997; 31: 411–417.
Mjör I A . Reaction patterns of dentin. In Thylstrup A, Leach S A, Qvist V (eds) Dentine and dentine reactions in the oral cavity. pp 27–31. Oxford: IRL Press Ltd, 1987.
Ogawa K, Yamashita Y, Ichijo T, Fusayama T . The ultrastructure and hardness of the transparent layer of human carious dentin. J Dent Res 1983; 62: 7–10.
Craig R G, Peyton F A . The microhardness of enamel and dentin. J Dent Res 1958; 37: 661–668.
Fusayama T, Okuse K, Hosoda H . Relationship between hardness, discoloration and microbial invasion in carious dentin. J Dent Res 1966; 45: 1033–1046.
Wirthlin M R Jr . Acid-reacting stains, softening and bacterial invasion in human carious dentin. J Dent Res 1970; 49: 42–46.
Goldstein R E, Parkins F M . Air-abrasive technology: its new role in restorative dentistry. J Am Dent Assoc 1994; 125: 551–557.
Ericson D, Zimmerman M, Raber H, Götrick B, Bornstein R, Thorell J . Clinical evaluation of efficacy and safety of a new method for chemo-mechanical removal of caries. Caries Res 1999; 33: 171–177.
Renson C E . Back to the future in cavity preparation. Dent Update 1995; 22: 93–95.
van der Veen M H, ten Bosch J J . The influence of mineral loss on the auto-fluorescent behaviour of in vitro demineralised dentine. Caries Res 1996; 30: 93–99.
Banerjee A . Applications of scanning microscopy in the assessment of dentine caries and methods for its removal. PhD Thesis, University of London, 1999.
Banerjee A, Boyde A . Autofluorescence and mineral content of carious dentine: scanning optical and backscattered electron microscopic studies. Caries Res 1998; 32: 219–226.
Garberoglio R, Brannström M . Scanning electron microscopic investigation of human dentinal tubules. Archs oral Biol 1976; 21: 355–362.
Fusayama T . Two layers of carious dentin: diagnosis and treatment. Op Dent 1979; 4: 63–70.
Author information
Authors and Affiliations
Additional information
Refereed Paper
Rights and permissions
About this article
Cite this article
Banerjee, A., Sherriff, M., Kidd, E. et al. A confocal microscopic study relating the autofluorescence of carious dentine to its microhardnes. Br Dent J 187, 206–210 (1999). https://doi.org/10.1038/sj.bdj.4800241
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.bdj.4800241