Abstract
A major regulator of lymphocyte survival and activation is the transcription factor nuclear factor-κB (NF-κB). Controlled activation of NF-κB is essential for the immune and inflammatory response as well as for cell proliferation and protection against apoptosis. The NEMO/IκB kinase (IKK) complex is the central integrator of most stimuli leading to NF-κB activation, but a detailed knowledge of the upstream events is available only for a limited number of stimuli. In particular, although most players have probably been identified, relatively little is known about the detailed molecular mechanisms involved in the cascade leading to NF-κB activation following engagement of the T-cell receptor by a foreign antigen. In this review, we discuss recent insights into this specific signal transduction cascade, and the way it is controlled both spatially and temporally.
Similar content being viewed by others
Introduction
Nuclear factor-κB (NF-κB) is the generic name of a family of transcription factors that act as dimers and regulate genes involved in the inflammatory and immune responses as well as in some aspects of cell growth, survival and differentiation. The aim of this review is to summarize our current knowledge of NF-κB activation in response to T-cell receptor (TCR) stimulation. The NF-κB family comprises five subunits: c-Rel, RelA (p65), RelB, NF-κB1 and NF-κB2, each encoded by a distinct locus. In most cells, the major proportion of NF-κB proteins resides in the cytoplasm in a latent state, sequestered by inhibitory proteins, called IκB's. The activation of NF-κB can be divided into two phases. The first phase involves cytoplasmic events leading to the activation of a kinase complex composed of three subunits: IκB kinase (IKK)α, IKKβ and NEMO/IKKγ. IKKα and IKKβ are catalytic kinase subunits, whereas NEMO is a structural and regulatory subunit. This kinase complex phosphorylates two serine residues in the N-terminal region of the IκBs, leading to their polyubiquitination and proteasome-mediated degradation.1 The main consequence of IκB degradation is the translocation of NF-κB dimers to the nucleus. Recently, a second NF-κB activation pathway (called the alternative pathway) based on regulated processing of NF-κB2/p100 precursor protein has been identified.2, 3 This pathway is involved in NF-κB activation in response to a specific set of stimuli that includes BAFF, LTβ and CD40L, and seems to be more specifically involved in B-cell maturation and lymphoid organ formation. Following stimulation, NF-κB2/p100 is partially degraded in a NIK (a member of the MAPKKK family) and IKKα-dependent manner, therefore, liberating p52/relB complexes which activate a subset of NF-κB target genes.4
Following these cytoplasmic events, the second phase occurs primarily in the nucleus and involves post-translational modifications of the NF-κB subunits, which are required to regulate NF-κB transcriptional activity.5, 6, 7 This two-step mechanism of NF-κB activation is common to all cell types and stimuli, however, TCR-mediated activation of NF-κB is characterized by the orchestration of the signaling process in spatially segregated domains. We will focus this review on the events that take place upstream of the IKK complex as the connection between TCR activation and specific nuclear modifications of NF-κB subunits is currently poorly understood.
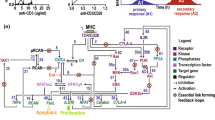
Several genetic studies have identified signaling components involved in the TCR to NF-κB pathway: ZAP-70,8 SLP-76,8, 9 PLCγ1,9 SAP,10 Fyn,10 PKCθ,11 Vav1,12 Bcl-10,13 Carma1,14, 15 MALT1/paracaspase16, 17 and RIP-2.18, 19 These molecules can play important roles at distinct times and places during the different phases of the NF-κB response in the course of TCR stimulation. Biochemical studies have established a putative chronological model of activation of these effectors. In this model, PKCθ acts upstream of a complex of three proteins (Carma1, Bcl10 and MALT1), referred as Carma1-Bcl10-MALT1 (CBM), in order to promote the activation of the NEMO/IKK complex. However, this mechanism is also spatially controlled and other players are needed for the recruitment of CBM into a region called the Supramolecular Activation Cluster (SMAC), for the regulation of CBM activity and also for the recruitment of IKK to the CBM complex (Figure 1).
A spatio-temporal model for TCR-induced NF-κB activation. (a) Stimulation of T cells via TCR/CD28 costimulation elicits sequential activation of two classes of protein tyrosine kinases: Src family kinases Lck and Fyn first phosphorylate the TCR, then ZAP-70 is recruited to the phosphorylated TCR. ZAP-70 then phosphorylates the adaptor proteins LAT and SLP76. These phosphorylations organize multimolecular complexes containing PLC-γ1 and the nucleotide exchange factor Vav1. Active PLC-γ1 generates IP3, which induces the release of Ca2+ from intracellular stores, and DAG, which stimulates PKCθ. (b) PKCθ is translocated into the IS through a mechanism requiring the activation of PI3K by the engagement of CD28, then PI3K induces PDK1 recruitment to the plasma membrane and PDK1 in turn phosphorylates PKCθ on threonine 538, a process required for the recruitment of PKCθ to the plasma membrane. This recruitment is also stimulated by Vav1 and by the engagement of the SLAM receptor through a pathway involving Fyn and SAP. Once in the IS, PKCθ is phosphorylated by Lck on tyrosine. The activation of PKCθ is essential for the release of WASP from WIP inhibition and subsequently for F-actin polymerisation. PKCθ also phosphorylates Moesin, a molecule involved in actin reorganization during vesicular trafficking to the plasma membrane. (c) Subsequently, PDK1 allows the direct binding of PKCθ to IKK and also the recruitment of Bcl10 and MALT1 to the lipid rafts through the phosphorylation of Carma1 by PKCθ. Caspase 8 is also required for TCR-induced NF-κB activation by facilitating the binding of IKK to the CBM complex. (d) Finally, PKCθ, the CBM and the IKK complexes are recruited to the TCR signaling complex in the IS. A poorly understood mechanism involving ubiquitination of NEMO and phosphorylation of IKKβ by TAK1 then leads to the activation of the IKK complex. Once activated, the IKK kinases phosphorylate the IκB inhibitors of NF-κB inducing their ubiquitination and degradation. Following this process, NF-κB translocates to the nucleus and activates its target genes. Finally, the termination of NF-κB involves specific mechanisms such as targeted degradation of signaling molecules (indicated for Bcl10 in the figure)
Proximal Events in TCR Signaling
In T lymphocytes, the primary activating signal is the recognition of suitable peptide – MHC ligands on the surface of antigen presenting cells (APC). Binding of MHC/antigen complexes by the TCR, or cross-linking by anti-TCR or anti-CD3 antibodies orchestrate intracellular protein tyrosine phosphorylation events. These events are sequentially activated by three families of protein tyrosine kinases: Src, Syk and Tec. Signals from other cell surface receptors, particularly CD28, cooperate with TCR signaling to produce full T-cell activation.20 The nature of this costimulation is difficult to ascertain. CD28 is engaged by its ligands B7-1 (CD80) and B7-2 (CD86). One model is that CD28 plays a role in augmenting signals delivered through the TCR rather than delivering a unique second signal, but this is still a matter of debate. Ultimately, these signals result in T-cell differentiation, entry into cycle, and the production of cytokines such as interleukin 2 (IL-2). Induction of IL-2 secretion is mediated by the binding of the inducible transcription factors AP-1, NF-AT and NF-κB to the promoter region of the IL-2 gene. These transcription factors are regulated by distinct mechanisms and while we have focused this review on NF-κB, the cooperative binding of all these factors is required for expression of IL-2 as well as of a number of other genes in response to TCR signaling.
Following the activation of the TCR/CD3, the Src family kinase Lck is recruited to the TCR complex via its association with CD4 or CD8 coreceptors. Lck phosphorylates conserved motives of the CD3 complex, the ITAMs, leading to the recruitment of ZAP-70, a 70 kDa protein tyrosine kinase of the Syk family. ZAP-70 in turn phosphorylates several substrates including the transmembrane adaptor molecule LAT and the cytosolic adaptor protein SLP-76. These proteins then serve as docking surfaces for other adaptors such as Grb2, GADS, the p85 regulatory subunit of phosphatidylinositol-3 kinase (PI3-K) and phospholipase Cγ. Grb2 may also mediate the activation of the Rho family GTPases Rac and Cdc42 by indirectly recruiting Vav, a member of the Dbl superfamily of Rac/Rho-specific guanine nucleotide exchange factors. This function is also carried out by SLP-76, which binds both Grb2 and Vav in response to TCR ligation. The association between tyrosine-phosphorylated SLP-76 and Vav seems to be required for NF-κB activation in response to coengagement of CD3 and CD28.8 In addition to its involvement in TCR-induced NF-κB activation, Vav is also important for NFAT, AP1, JNK activation and for sustained Ca2+ signaling.12, 21 Recent data suggest that Vav associates with IKKα, targets it to the membrane and activates it in response to CD28 stimulation.22 Vav is also required for translocation of PKCθ to the immune synapse in response to CD3/CD28 costimulation,23 although the physiological importance of this event is still unknown.
A Central Role for PKCθ
Emerging evidence suggests that PKCθ, a novel Ca2+-independent PKC isoform essentially expressed in skeletal muscle and lymphoid tissue (but not in B cells) is required for TCR-mediated activation of mature T cells,24 and is selectively implicated in NF-κB and JNK activation. The activity of PKCθ is essentially regulated by membrane recruitment and conformational changes. Among the several PKC isoenzymes expressed in T cells, PKCθ is the only one to be translocated to the plasma membrane following TCR activation.25 This membrane relocalization of PKCθ is associated with its redistribution into insoluble membrane microdomains called ‘rafts’, where a variety of intracellular molecules, such as Lck, ZAP-70, LAT have been shown to accumulate. The presence of PKCθ in these structures is necessary for NF-κB activation,26 and has been associated with three different, although not mutually exclusive, mechanisms:
-
a)
Vav and Lck have been shown to be required for the membrane relocalization of PKCθ.26, 27 In addition, the raft-resident fraction of PKCθ is physically associated with Lck, therefore, facilitating its phosphorylation by Lck on Tyr,90 an important event for NF-κB activation.
-
b)
The membrane recruitment and activation of PKCθ in response to CD28 engagement is also mediated by a PI3K- and Vav/Rac-dependent but PLC independent pathway. Activation of PI3K induces PDK1 recruitment to the plasma membrane and PDK1 in turn phosphorylates PKCθ on threonine 538, which induces its recruitment to the membrane lipid rafts.23, 28, 29
-
c)
Recently, it has been shown using a genetic approach that SAP, the protein mutated in X-linked lymphoproliferative disease (XLP), is recruited to the TCR contact site and plays a critical role in TCR-stimulated PKCθ and Bcl10 recruitment to raft fractions.10 These recruitments are stimulated by the engagement of the SLAM family receptors and are independent of Vav and Akt.10 Although study of the role of Fyn in PKCθ regulation has led to conflicting results,30 Fyn-deficient cells exhibit defects similar to those seen in SAP-deficient cells.10 Furthermore, this study suggests that a SAP/Fyn-mediated pathway is involved in the fine-tuned regulation of a subset of NF-κB family members.
Therefore, signals that emanate from the TCR (Lck), from CD28 (PI3K/Akt) and from SLAM (SAP/Fyn) are required for an efficient translocation of PKCθ to the lipid rafts and for the TCR-mediated activation of NF-κB. Interestingly, PKCθ not only translocates into membrane lipid rafts, but also into the central region of the SMAC (see ‘The immunological synapse controls TCR signaling’).
Regarding its conformational changes, it has been shown that the amino-terminal region of PKCθ is responsible for inactivation of its catalytic region under nonstimulated conditions. A constitutively active PKCθ can thus be generated by replacing a critical Ala (A148) by Glu in the amino-terminal region (PKCθ A/E), therefore, leaving the catalytic site free for interaction with substrates.31, 32 Overexpression assays and genetic studies have demonstrated the selective implication of PKCθ in mediating NF-κB activation via the TCR pathway. Indeed, overexpression of a constitutively active form of PKCθ is able to activate NF-κB 33, 34, 35 in a T-cell and stimulus-specific manner: this constitutive form of PKCθ is not active in nonlymphoid cells, and a kinase-deficient form of it inhibits TCR-induced, but not TNFα or IL-1-induced NF-κB activation.33, 35
Inactivation of the pkcθ gene in the mouse confirmed that this kinase is a critical participant in the NF-κB pathway leading to IL-2 transcription in mature but not immature T lymphocytes.11 However, these results have been recently challenged by a second study,36 in which NFAT transactivation following CD3/CD28 engagement in mature T cells derived from PKCθ knockout mice was completely abrogated, but NF-κB transactivation was only slightly reduced. The reasons for these discrepancies might involve differences in the inactivation strategies, but further experiments are clearly required to resolve this issue. The generation of PKCθ deficient T-cell lines should be a powerful tool to further examine these points.
The induction of NF-κB by PKCθ has been shown to be selectively mediated by IKKβ.33, 35 Therefore, PKCθ is not involved in the so-called alternative pathway of activation of NF-κB.37
Recently, it was shown that the linker region of Carma1 (located between the coiled coil and PDZ domains) is phosphorylated by PKCθ after lymphocyte activation.38 Another study reported that engagement of the B-cell antigen receptor leads to the phosphorylation of the linker region of Carma1 by PKCβ.39 These phosphorylation events are the critical link that controls the assembly of the CBM complex by regulating the accessibility of Carma1 for interaction with Bcl10/MALT1 and thereby NF-κB signalling.
In addition, several studies suggest that PKCθ may contribute to the dynamic organization of the immune synapse by regulating the phosphorylation of actin-interacting proteins.40 Moesin, a member of the ERM family of actin cytoskeletal membrane linkage proteins, is phosphorylated in vitro by PKCθ on Thr,558 in the actin-binding domain, thus regulating the interaction of Moesin with the actin cytoskeleton. It has also been shown that PKCθ is implicated in the mechanism of recruitment in the immune synapse of WASP, the Wiskott-Aldrich syndrome protein.41 WASP plays a critical role in T-cell activation and actin reorganization. It is kept in an inactive conformation through its association with WASP interacting protein (WIP). Upon TCR engagement, the WIP–WASP complex is recruited to ZAP-70 in the immune synapse by the adaptor protein CrkL, and following PKCθ-mediated phosphorylation of WIP, WASP is released from the complex. The dissociation of this complex is important for F-actin polymerization and IL-2 synthesis.
The CBM Complex Activates the NEMO/IKK Complex
Carma1, Bcl10 and MALT1 play an important and previously unforeseen role in antigen receptor-induced NF-κB activation downstream of PKC, but relatively little is known about the precise function of these molecules, nor how they connect to upstream PKC's. Bcl10 was originally identified as a target of a recurrent chromosomal translocation t(1;14)(p22;q32) associated with aggressive MALT lymphomas. Several groups concurrently identified Bcl10 as a novel member of a family of proteins with a caspase recruitment domain (CARD).42 As several proteins containing a CARD motif are involved in controlling apoptosis, it was initially suggested that the function of Bcl10 was to regulate proapoptotic signaling pathways. Bcl10 deficient mice did not confirm these predictions but rather unraveled an unexpected role of Bcl10 in humoral and cellular immune responses, antigen-induced proliferation and activation of NF-κB in T and B cells.13 In addition, Bcl10-deficient mice exhibit defects in neural tube closure. Bcl10 is essential not only for the function but also for the maturation of B cells,43 and its impairment results in a reduction of the number of follicular and marginal zone B cells. The signaling defect in Bcl10−/− cells cannot be overcome by treatment with direct PKC activators (such as PMA or PMA/ionomycin). Thus, these findings identify Bcl10 as a key player in antigen receptor-induced NF-κB activation downstream of PKCs.
Carma1 (CARD11/Bimp3) was identified by several groups as a Bcl10 interacting protein44, 45, 46, 47 and represents the only lymphocyte-specific member in a family of membrane-associated guanylate kinase (MAGUK) proteins that interact with Bcl10 through CARD–CARD interactions. Like other proteins of the same family, Carma1 is characterized by the presence of an amino-terminal CARD domain, followed by a coiled coil motif and a MAGUK motif containing a PDZ (a motif present in the Psd-95, Dlg (discs large) and ZO-1 (zonula occludens-1) proteins), a Src homology-3 (SH3) domain and a guanylate domain (GUK). Several lines of evidence support a role for Carma1, upstream of Bcl10 in TCR-induced NF-κB activation. First, overexpression of Carma1 induces NF-κB activation as well as Bcl10 phosphorylation.45 This activation does not take place in Bcl10−/− cells, suggesting that Carma1 is an upstream regulator of Bcl10.46 Second, Carma1 is constitutively associated with lipid rafts, interacts with Bcl10 and the TCR complex upon TCR stimulation 48 and is required for the recruitment of Bcl10 to clustered TCR complexes.14 The interaction of Carma1 with Bcl10 and its effect on NF-κB activation is prevented by a mutation in its CARD domain (L39R).48 Third, inactivation of Carma1 in cell lines by somatic mutagenesis or RNA interference results in selectively impaired activation of NF-κB by the TCR.49, 50 The defect in Carma1-deficient T cells was found to lie downstream of PKCθ, and it was further demonstrated that Carma1 connects PKCθ to Bcl10.50 Fourth, genetic inactivation of Carma1 in the mouse results in a complete block in T- and B-cell immunity, including inactivation of the NF-κB and JNK pathways in response to TCR and BCR crosslinking.14, 15, 51, 52 Knockout mice exhibit a profound defect in the development and/or survival of B1 and NK cells, and a complete loss of B-cell response to LPS, anti-IgM, CD40L and antigen, as well as a decreased T-cell response to the TCR.51 Fifth, constitutively active PKCθ does not induce NF-κB activation in Carma1-deficient cells.50 Sixth, PKCθ phosphorylates Carma1 and mutation of the phosphorylation sites completely abolish the function of Carma1.38 Altogether, these findings demonstrate that Carma1 plays a crucial role downstream of PKCθ and upstream of Bcl10 in the activation of the NF-κB pathway in response to TCR stimulation.
Beside Bcl10 and Carma1, the human paracaspase MALT1 has been recently identified as a caspase like protein involved in NF-κB activation.53, 54 The molecular structure of MALT1 is characterized by an N-terminal death domain (DD) followed by two immunoglobulin (Ig)-like domains and a C-terminal caspase-like domain. The C-terminal caspase-like domain characterizes MALT1 as a member of the paracaspase family, an evolutionary conserved family of unknown function.54 Like Bcl10, the gene encoding this protein is involved in a chromosomal translocation seen in some MALT lymphomas. This translocation (t(11;18) (q21;q21)) encodes a chimeric protein consisting of the N-terminal portion of the antiapoptotic protein c-IAP2 linked to the C terminus of MALT1, which is capable of activating NF-κB.54 In addition to their common implication in the pathogenesis of MALT lymphoma, Bcl10 and MALT1 physically and functionally interact to synergistically activate NF-κB.53, 54 Both the ability to bind Bcl10 and an intact caspase-like domain are required for the synergistic activation of NF-κB by Bcl10 and MALT1.53, 54 Two studies have reported the analysis of MALT1 deficient mice, although with conflicting results.16, 54 While they both show that in contrast to Bcl10 deficiency, inactivation of MALT1 does not cause defects during neurodevelopment, they present conflicting results regarding the immune system. In one paper, the phenotype of the mice was reminiscent of that of Carma1 and Bcl10 deficient mice, with profound defects in proliferation, cytokine production and NF-κB activation in antigen-stimulated T cells, and defects in BCR, LPS and CD40-induced stimulation of B cells.16 However, these mice demonstrated intact JNK activation after T- and B-cell stimulation. In the second paper, the T-cell defect was nearly identical (despite impairment of the JNK pathway, which was a bit unexpected as this pathway was not affected by the absence of the upstream molecule Bcl10), but the B-cell situation was different: MALT1 was required for B1 and marginal zone B-cell development, but dispensable for anti-IgM, LPS and anti-CD40 induced proliferation of mature splenic B2 cells and activation of NF-κB in response to BCR stimulation. This discrepancy might be due to different strategies used to inactivate the MALT1 gene. Nevertheless, in the two papers all immunoglobulin isotypes tested were found to be reduced and MALT1−/− mice failed to mount an immune response after immunization.17 A breakthrough in the field came with a recent paper from the Dixit laboratory,55 showing that Bcl10 mediates NEMO polyubiquitination (through Lys63-linked chains) in a MALT1-dependent manner. This step seems important for the ‘activation’ of NEMO, since a mutant form of this protein that cannot be ubiquitinated inhibits Bcl10-induced NF-κB activation. In parallel, others have shown that TRAF6, an adaptor protein already known to be involved in NF-κB-activation by stimuli such as IL-1, and recently demonstrated to function as an ubiquitin ligase catalyzing the formation of K63-linked polyubiquitin chains,56, 57 mediates IKK activation by Bcl10 and MALT1 in conjunction with the TAK1 kinase, a member of the MAPKKK family58 (see Chen et al., this issue).
Regulation of the CBM Complex Activity and Recruitment
The mechanism by which Bcl10 activity is regulated in the CBM complex is not fully understood. A number of studies suggest that this regulation involves phosphorylation events, but the positive or negative effect of these events is still a matter of debate. What is clear is that overexpression of Carma1 induces Bcl10 phosphorylation as well as NF-κB activation.45 This suggest that Carma1 connects Bcl10 to its potential(s) kinase(s) or that it activates a Bcl10-associated kinase. Indeed it was demonstrated that Carma1 connects the protein kinase PKCθ to Bcl10 to positively regulate NF-κB activation,50 although it was not demonstrated that PKCθ directly phosphorylates Bcl10.50 Consistently, SAP-deficient T cells showed a defect in PKCθ localization in addition to an inefficient recruitment and phosphorylation of Bcl10.10 Another kinase, RIP2 has also been suggested to regulate Bcl10 signaling.59 RIP2 (also known as Rick, CARDIAK or CCK) is a serine/threonine kinase that contains a CARD domain at its carboxy terminus, and shares sequence similarity with RIP, an essential kinase for NF-κB activation by TNF.60 Genetic studies have demonstrated that RIP2 is required for optimal activation of NF-κB and T-cell proliferation upon TCR stimulation.18, 19 Indeed, RIP2 deficient T cells show severely reduced proliferation upon CD3 or CD3/CD28 stimulation, as well as a reduced NF-κB activation and IL-2 production. They also show impaired differentiation to Th1 cells. A recent paper suggests that RIP2 associates with and phosphorylates Bcl10 in response to TCR engagement.59
In addition to the positive effects of Bcl10 on NF-κB signaling in response to TCR stimulation, Bcl10 phosphorylation events have also been implicated in the negative regulation of this pathway. Recently, it has been shown that Bcl10 becomes phosphorylated and is subsequently degraded through the lysosome pathway in response to PMA in a NEMO-independent but PKCθ-dependent manner.61 Some unexpected results showed that Bcl10 can be phosphorylated by the NEMO/IKK complex itself and that this event may contribute to negatively regulate TCR-induced NF-κB signaling cascade.62 Since the activation of NF-κB in response to TCR engagement requires the activation of the NEMO/IKK complex by the CBM complex, the question is where and how do these complexes interact. One kinase upstream of PKCs is 3-phosphoinositide-dependent kinase (PDK-1), whose activity is regulated by PI3 K. Recently, Lee and co-workers have shown that PDK1 is involved not only in the regulation of PKCθ activity but also in the recruitment of PKCθ and Carma1 to the lipid rafts.28 Furthermore, they have defined two independent functions of PDK1: the pool of PDK1 associated with PKCθ recruits the IKK complex to the lipid rafts whereas the pool of PDK1 associated with Carma1 recruits Bcl10 and MALT1. Another study has demonstrated that FADD and caspase 8 bind to CBM to allow the recruitment and activation of IKK.63 Thus caspase 8 may facilitate the oligomerization of CBM which has been shown to be activated at least partially through this process.
The Immunological Synapse Controls TCR Signaling
While the complex sequence of events during which T cells recognize foreign peptides bound to major histocompatibility complex begins to emerge, less is known about the spatial and dynamic characteristics of these events. Over the last several years, new approaches involving a variety of cell imaging techniques have been developed to address this issue.64 Using these techniques, it has become clear that intracellular signaling molecules, adaptors and cytoskeletal proteins appear to be associated with highly spatially and temporally organized structures. These highly organized molecular assemblies have been termed Supra-Molecular Activation Clusters (SMACs). The specialized area formed at the contact site between T cells and antigen-presenting cells (APC) has been generically termed the ‘Immunological synapse’ (IS). The time-dependent molecular composition and the functional role of the SMAC and the IS are under intense investigation. For example, the rapidity of the recruitment of intracellular proteins to the T-cell-APC contact site, under conditions of complete or partial activation, may be correlated with the specific function of a signaling molecule. In addition, the spatial segregation of intracellular proteins in distinct areas of the SMAC (central-SMAC (c-SMAC) and peripheral-SMAC (p-SMAC)) correlates with their importance in the activation pathway. For example, it has been shown that TCR-mediated tyrosine kinase signaling occured primarily at the periphery of the synapse and is largely terminated before mature IS formation.65
Over the past few years, several laboratories have shown that the signaling components of TCR-mediated NF-κB activation are localized in the IS. Although the relationship between the IS and membrane lipid rafts remains unknown, several studies have shown that PKCθ colocalizes with rafts to the central region of the IS.26, 27 In naive CD4T cells, TCR-mediated signals are sufficient to induce the capping of PKCθ to the region of TCR engagement by its ligand, however, examination of the morphology of the IS revealed that CD28-mediated signals are required for the localization of PKCθ to the c-SMAC of the synapse.27
Carma1 is a member of the membrane-associated guanylate kinase (MAGUK) and a general feature of these proteins is their association with membrane proteins. Carma1 is constitutively present in lipid rafts and is recruited to the IS where it colocalizes with Bcl10 following TCR stimulation.48, 66 The recruitments of Carma1 and Bcl10 are dependent upon the integrity of the TCRβ chain. Indeed, it has been reported that a mutant of the transmembrane and cytoplasmic domains of the TCRβ chain is impaired in recruiting Carma1 and Bcl10 and consequently in activating NF-κB.67 In addition to being important for the recruitment of Bcl10 to the IS, Carma1 may also be important for the recruitment of PKCθ.66 However, another study did not reach the same conclusion but rather concluded that recruitment of PKCθ to central and peripheral IS occurred normally in Carma1 deficient T cells.68 In addition, two other components, SAP and the receptor SLAM, which are also recruited to the IS, are required to favor the presence of PKCθ in the IS.10
Finally, the core NF-κB activating machinery, the NEMO/IKK complex, is also recruited to the IS and can be co-precipitated with the TCR following stimulation.69 Moreover, artificial targeting of NEMO to the IS is sufficient to specifically induce NF-κB activation in response to TCR stimulation. This result is also supported by the recent finding that Carma1 is essential for recruitment of IKK into c-SMAC and for its activation.66, 68
It is now well documented that the IS is a specialized domain where sustained engagement and signaling of TCR occur. Increasing evidence indicates that the IS may play a dual role in T-cell activation, being not only the area where T-cell activation occurs but also the place were key activation molecules are degraded. Indeed the IS has recently been identified as the area where TCR-induced protein ubiquitination occurs.70, 71 Whether or not signaling components of the TCR-mediated NF-κB pathway are also degraded in the IS remains an open question.
Concluding Remarks
In lymphocytes, NF-κB controls the expression of multiple genes essential for the immune response. The signaling pathways leading to the activation of NF-κB in this context have been the subject of intense studies. A plausible cascade of events in T cells is as follows: PDK1 recruits PKCθ and Carma1 to the IS.28 Then, Carma1 is phosphorylated by PKCθ,38 therefore, enabling the association with Bcl10/MALT1 to form the CBM complex. The NEMO/IKK complex is then recruited to Carma1 with the help of caspase 8.63 The following step is the ‘activation’ of Bcl10 by a putative kinase. Then, Bcl10 activates the NEMO/IKK complex through a MALT1-dependent ubiquitination mechanism.55 Finally, the selective termination of NF-κB activation involves active mechanisms such as the degradation of key signaling molecules. This mechanism should protect cells from the deleterious effect of chronic activation or should induce T-cell anergy. Indeed, it has been shown that sustained signaling through Ca2+ and calcineurin results in lysosomal degradation of PKCθ and PLC-γ1, a process involved in T-cell unresponsiveness (anergy) and tolerance.72
This signaling pathway is observed in ‘mature’ T-cells and represents an important step for T-cell proliferation in response to antigens and pathogens. However, different stimuli leading to NF-κB activation have been shown to be involved at distinct steps of T-cell differentiation.73 These signaling events are initiated by T-cell specific differentiation products, for example, the pre-T-cell receptor (pre-TCR) and mature TCRαβ, and play a critical role in T-cell lineage commitment and T-cell development and functions. In parallel, a specific role for signaling by a member of the Notch family, Notch3, has been demonstrated at the pre-TCR checkpoint.73 Indeed, the in vivo expression of a constitutively activated form of Notch3 in the thymus is responsible for NF-κB activation in all thymocyte subsets and peripheral T cells.74 However, it is unclear whether the pre-TCR and Notch activate NF-κB through a signaling pathway identical to the one described for mature T cells. Recently, it has been reported that PKCθ is a downstream target of Notch3 signaling and that its activation and membrane translocation require a functional pre-TCR in order to trigger NF-κB activation.75 Clearly, further work will be required to determine whether the signaling intermediates relevant to NF-κB activation in mature T cells are also implicated in T-cell differentiation.
In conclusion, the use of mouse and cellular genetic models as well as biochemical studies have established a coherent picture of the molecular events leading to the activation of NF-κB following the engagement of the TCR by an antigenic peptide. A better knowledge of the specific regulators controlling this pathway would help to design immunosuppressive drugs able to target NF-κB activation, which is often found to favor the appearance and development of lymphoid malignancies.
Abbreviations
- NF-κB:
-
nuclear factor-κB
- IKK:
-
IκB kinase
- TCR:
-
T-cell receptor
- APC:
-
antigen presenting cell
- CARD:
-
caspase recruitment domain
- SMAC:
-
supra molecular activation complex
- IS:
-
immunological synapse
- CBM:
-
Carma1-Bcl10-MALT1
References
Hayden MS and Ghosh S (2004) Signaling to NF-kappaB. Genes Dev. 18: 2195–2224
Ghosh S and Karin M (2002) Missing pieces in the NF-kappaB puzzle. Cell 109: S81–S96
Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, Chen Y, Hu Y, Fong A, Sun SC and Karin M (2001) Activation by IKKalpha of a second, evolutionary conserved, NF-kappaB signaling pathway. Science 293: 1495–1499
Xiao G, Harhaj EW and Sun SC (2001) NF-kappaB-inducing kinase regulates the processing of NF-kappaB2 p100. Mol. Cell 7: 401–409
Mattioli I, Sebald A, Bucher C, Charles RP, Nakano H, Doi T, Kracht M and Schmitz ML (2004) Transient and selective NF-kappaB p65 serine 536 phosphorylation induced by T cell costimulation is mediated by IkappaB kinase beta and controls the kinetics of p65 nuclear import. J. Immunol. 172: 6336–6344
Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W and Haegeman G (2003) Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J. 22: 1313–1324
Zhong H, Voll RE and Ghosh S (1998) Phosphorylation of NF-kappaB p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol. Cell 1: 661–671
Herndon TM, Shan XC, Tsokos GC and Wange RL (2001) ZAP-70 and SLP-76 regulate protein kinase C-theta and NF-kappaB activation in response to engagement of CD3 and CD28. J. Immunol. 166: 5654–5664
Dienz O, Moller A, Strecker A, Stephan N, Krammer PH, Droge W and Schmitz ML (2003) Src homology 2 domain-containing leukocyte phosphoprotein of 76 kDa and phospholipase C gamma 1 are required for NF-kappaB activation and lipid raft recruitment of protein kinase C theta induced by T cell costimulation. J. Immunol. 170: 365–372
Cannons JL, Yu LJ, Hill B, Mijares LA, Dombroski D, Nichols KE, Antonellis A, Koretzky G, Gardner K and Schwartzberg PL (2004) SAP regulates Th2 differentiation and PKC-theta-mediated activation of NF-kappaB1. Immunity 21: 693–706
Sun Z, Arendt CW, Ellmeier W, Schaeffer EM, Sunshine MJ, Gandhi L, Annes J, Petrzilka D, Kupfer A, Schwartzberg PL and Littman DR (2000) PKC-theta is required for TCR-induced NF-kappaB activation in mature but not immature T lymphocytes. Nature 404: 402–407
Costello PS, Walters AE, Mee PJ, Turner M, Reynolds LF, Prisco A, Sarner N, Zamoyska R and Tybulewicz VLJ (1999) The Rho-family GTP exchange factor Vav is a critical transducer of T cell receptor signals to the calcium, ERK, and NF-kappaB pathways. Proc. Natl. Acad. Sci. USA 96: 3035–3040
Ruland J, Duncan GS, Elia A, del Barco Barrantes I, Nguyen L, Plyte S, Millar DG, Bouchard D, Wakeham A, Ohashi PS and Mak TW (2001) Bcl10 is a positive regulator of antigen receptor-induced activation of NF-kappaB and neural tube closure. Cell 104: 33–42
Egawa T, Albrecht B, Favier B, Sunshine MJ, Mirchandani K, O’Brien W, Thome M and Littman DR (2003) Requirement for CARMA1 in Antigen Receptor-Induced NF-kappaB Activation and Lymphocyte Proliferation. Curr. Biol. 13: 1252–1258
Jun JE, Wilson LE, Vinuesa CG, Lesage S, Blery M, Miosge LA, Cook MC, Kucharska EM, Hara H, Penninger JM, Domashenz H, Hong NA, Glynne RJ, Nelms KA and Goodnow CC (2003) Identifying the MAGUK protein Carma-1 as a central regulator of humoral immune responses and atopy by genome-wide mouse mutagenesis. Immunity 18: 751–762
Ruefli-Brasse AA, French DM and Dixit VM (2003) Regulation of NF-kappaB-dependent lymphocyte activation and development by paracaspase. Science 302: 1581–1584
Ruland J, Duncan GS, Wakeham A and Mak TW (2003) Differential requirement for MALT1 in T and B cell antigen receptor signaling. Immunity 19: 749–758
Chin AI, Dempsey PW, Bruhn K, Miller JF, Xu Y and Cheng G (2002) Involvement of receptor-interacting protein 2 in innate and adaptive immune responses. Nature 416: 190–194
Kobayashi K, Inohara N, Hernandez LD, Galan JE, Nunez G, Janeway CA, Medzhitov R and Flavell RA (2002) RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416: 194–199
Kane LP, Lin J and Weiss A (2002) It’s all Rel-ative: NF-kappaB and CD28 costimulation of T-cell activation. Trends Immunol. 23: 413–420
Cao Y, Janssen EM, Duncan AW, Altman A, Billadeau DD and Abraham RT (2002) Pleiotropic defects in TCR signaling in a Vav-1-null Jurkat T-cell line. EMBO J. 21: 4809–4819
Piccolella E, Spadaro F, Ramoni C, Marinari B, Costanzo A, Levrero M, Thomson L, Abraham RT and Tuosto L (2003) Vav-1 and the IKK a subunit of IkappaB kinase functionally associate to induce NF-kappaB activation in response to CD28 engagement. J. Immunol. 170: 2895–2903
Villalba M, Bi K, Hu J, Altman Y, Bushway P, Reits E, Neefjes J, Baier G, Abraham RT and Altman A (2002) Translocation of PKCtheta in T cells is mediated by a nonconventional, PI3-K- and Vav-dependent pathway, but does not absolutely require phospholipase C. J. Cell Biol. 157: 253–263
Sedwick CE and Altman A (2002) Perspectives on PKCtheta in T cell activation. Mol. Immunol. 41: 675–686
Monks CR, Kupfer H, Tamir I, Barlow A and Kupfer A (1997) Selective modulation of protein kinase C-theta during T-cell activation. Nature 385: 83–86
Bi K, Tanaka Y, Coudronniere N, Sugie K, Hong S, van Stipdonk MJ and Altman A (2001) Antigen-induced translocation of PKC-theta to membrane rafts is required for T cell activation. Nature Immunol. 2: 556–563
Huang J, Lo PF, Zal T, Gascoigne NR, Smith BA, Levin SD and Grey HM (2002) CD28 plays a critical role in the segregation of PKCtheta within the immunologic synapse. Proc. Natl. Acad. Sci. USA 99: 9369–9373
Lee KY, D’Acquisto F, Hayden MS, Shim JH and Ghosh S (2005) PDK1 nucleates T cell receptor-induced signaling complex for NF-kappaB activation. Science 308: 114–118
Villalba M, Coudronniere N, Deckert M, Teixeiro E, Mas P and Altman A (2000) A novel functional interaction between Vav and PKC-theta is required for TCR-induced T cell activation. Immunity 12: 151–160
Ron D, Napolitano EW, Voronova A, Vasquez NJ, Roberts DN, Calio BL, Caothien RH, Pettiford SM, Wellik S, Mandac JB and Kauvar LM (1999) Direct interaction in T-cells between PKCtheta and the tyrosine kinase p59fyn. J. Biol. Chem. 274: 19003–19010
Baier-Bitterlitch G, Uberall F, Bauer B, Fresser F, Watchter H, Grunicke H, Utermann G, Altman A and Baier G (1996) Protein kinase C-theta isoenzyme selective stimulation of the transcription factor complex AP-1 in T lymphocytes. Mol. Cell. Biol. 16: 1842–1850
Meller N, Liu YC, Collins TL, Bonnefoy-Berard N, Baier G, Isakov N and Altman A (1996) Direct interaction between protein kinase Ctheta (PKCtheta) and 14-3-3 tau in T cells: 14-3-3 overexpression results in inhibition of PKCtheta translocation and function. Mol. Cell. Biol. 16: 5782–5791
Coudronniere N, Villalba M, Englund N and Altman A (2000) NF-kappaB activation induced by T cell receptor/CD28 costimulation is mediated by protein kinase C-theta. Proc. Natl. Acad. Sci. USA 97: 3394–3399
Khoshnan A, Bae D, Tindell CA and Nel AE (2000) The physical association of protein kinase Ctheta with a lipid raft-associated inhibitor of kappaB factor kinase (IKK) complex plays a role in the activation of the NF-kappaB cascade by TCR and CD28. J. Immunol. 165: 6933–6940
Lin X, O’Mahony A, Mu Y, Geleziunas R and Greene WC (2000) Protein kinase C-theta participates in NF-kappaB activation induced by CD3-CD28 costimulation through selective activation of IkappaB kinasebeta. Mol. Cell. Biol. 20: 2933–2940
Pfeifhofer C, Kofler K, Gruber T, Tabrizi NG, Lutz C, Maly K, Leitges M and Baier G (2003) Protein kinase Ctheta affects Ca2+ mobilization and NFAT cell activation in primary mouse T cells. J. Exp. Med. 197: 1525–1535
Li Y, Sedwick CE, Hu J and Altman A (2005) Role for protein kinase Ctheta (PKCtheta) in TCR/CD28-mediated signaling through the canonical but not the non-canonical pathway for NF-kappaB activation. J. Biol. Chem. 280: 1217–1223
Matsumoto R, Wang D, Blonska M, Li H, Kobayashi M, Pappu B, Chen Y, Wang D and Lin X (2005) Phosphorylation of Carma1 plays a critical role in T cell receptor-mediated NF-kappaB activation. Immunity 23: 575–585
Sommer K, Guo B, Pomerantz JL, Bandaranayake AD, Moreno-Garcia ME, Ovechkina YL and Rawlings DJ (2005) Phosphorylation of the Carma1 linker controls NF-kappaB activation. Immunity 23: 561–574
Thome M (2003) The immunological synapse and actin assembly: a regulatory role for PKCtheta. Dev. Cell 4: 3–5
Sasahara Y, Rachid R, Byrne MJ, de la Fuente MA, Abraham RT, Ramesh N and Geha RS (2002) Mechanism of recruitment of WASP to the immunological synapse and of its activation following TCR ligation. Mol. Cell 10: 1269–1281
Thome M and Tschopp J (2003) TCR-induced NF-kappaB activation: a crucial role for Carma1, Bcl10 and MALT1. Trends Immunol. 24: 419–424
Xue L, Morris SW, Orihuela C, Tuomanen E, Cui X, Wen R and Wang D (2003) Defective development and function of Bcl10-deficient follicular, marginal zone and B1 B cells. Nat. Immunol. 4: 857–865
Bertin J, Wang L, Guo Y, Jacobson MD, Poyet JL, Srinivasula SM, Merriam S, DiStefano PS and Alnemri ES (2001) CARD11 and CARD14 are novel caspase recruitment domain (CARD)/membrane-associated guanylate kinase (MAGUK) family members that interact with BCL10 and activate NF-kappaB. J. Biol. Chem. 276: 11877–11882
Gaide O, Martinon F, Micheau O, Bonnet D, Thome M and Tschopp J (2001) Carma1, a CARD-containing binding partner of Bcl10, induces Bcl10 phosphorylation and NF-kappaB activation. FEBS Lett. 496: 121–127
McAllister-Lucas LM, Inohara N, Lucas PC, Ruland J, Benito A, Li Q, Chen S, Chen FF, Yamaoka S, Verma IM, Mak TW and Nunez G (2001) Bimp1, a MAGUK family member linking PKC activation to Bcl10-mediated NF-kappaB induction. J. Biol. Chem. 276: 30589–30597
Wang L, Guo Y, Huang WJ, Ke X, Poyet JL, Manji GA, Merriam S, Glucksmann MA, DiStefano PS, Alnemri ES and Bertin J (2001) Card10 is a novel caspase recruitment domain/membrane-associated guanylate kinase family member that interacts with BCL10 and activates NF-kappaB. J. Biol. Chem. 276: 21405–21409
Gaide O, Favier B, Legler DF, Bonnet D, Brissoni B, Valitutti S, Bron C, Tschopp J and Thome M (2002) CARMA1 is a critical lipid raft-associated regulator of TCR-induced NF-kappaB activation. Nat. Immunol. 3: 836–843
Pomerantz JL, Denny EM and Baltimore D (2002) CARD11 mediates factor-specific activation of NF-kappaB by the T cell receptor complex. EMBO J. 21: 5184–5194
Wang D, You Y, Case SM, McAllister-Lucas LM, Wang L, DiStefano PS, Nunez G, Bertin J and Lin X (2002) A requirement for CARMA1 in TCR-induced NF-kappaB activation. Nat. Immunol. 3: 830–835
Hara H, Wada T, Bakal C, Kozieradzki I, Suzuki S, Suzuki N, Nghiem M, Griffiths EK, Krawczyk C, Bauer B, D’Acquisto F, Ghosh S, Yeh WC, Baier G, Rottapel R and Penninger JM (2003) The MAGUK family protein CARD11 is essential for lymphocyte activation. Immunity 18: 763–775
Jun JE and Goodnow CC (2003) Scaffolding of antigen receptors for immunogenic versus tolerogenic signaling. Nat. Immunol. 4: 1057–1064
Lucas PC, Yonezumi M, Inohara N, McAllister-Lucas LM, Abazeed ME, Chen FF, Yamaoka S, Seto M and Nunez G (2001) Bcl10 and MALT1, independent targets of chromosomal translocation in MALT lymphoma, cooperate in a novel NF-kappaB signaling pathway. J. Biol. Chem. 276: 19012–19019
Uren AG, O’Rourke K, Aravind LA, Pisabarro MT, Seshagiri S, Koonin EV and Dixit VM (2000) Identification of paracaspases and metacaspases: two ancient families of caspase-like proteins, one of which plays a key role in MALT lymphoma. Mol. Cell 6: 961–967
Zhou H, Wertz I, O’Rourke K, Ultsch M, Xiao W and Dixit VM (2004) Bcl10 activates NF-kappaB pathway through paracaspase/MALT1 dependent ubiquitination of NEMO/IKKgamma. Nature 427: 167–171
Chen ZJ (2005) Ubiquitin signalling in the NF-kappaB pathway. Nat. Cell. Biol. 7: 758–765
Deng L, Wang C, Spencer E, Yang L, Braun A, You J, Slaughter C, Pickart C and Chen ZJ (2000) Activation of the IkappaB kinase complex by TRAF6 requires a dimeric ubiquitin-conjugating enzyme complex and a unique polyubiquitin chain. Cell 103: 351–361
Sun L, Deng L, Ea CK, Xia ZP and Chen ZJ (2004) The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol. Cell 14: 289–301
Ruefli-Brasse AA, Lee WP, Hurst S and Dixit VM (2004) Rip2 participates in Bcl10 signaling and T-cell receptor-mediated NF-kappaB activation. J. Biol. Chem. 279: 1570–1574
Kelliher MA, Grimm S, Ishida Y, Kuo F, Stanger BZ and Leder P (1998) The death domain kinase RIP mediates the TNF-induced NF-kappaB signal. Immunity 8: 297–303
Scharschmidt E, Wegener E, Heissmeyer V, Rao A and Krappmann D (2004) Degradation of Bcl10 induced by T-cell activation negatively regulates NF-kappaB signaling. Mol. Cell. Biol. 24: 3860–3873
Lobry C, Lopez T, Israël A and Weil R . unpublished results
Su H, Bidère N, Zheng L, Cubre A, Sakai K, Dale J, Salmena L, Hakem R, Straus S and Lenardo M (2005) Requirement for caspase-8 in NF-kappaB activation by antigen receptor. Science 307: 1465–1468
Kupfer A and Kupfer H (2003) Imaging immune cell interactions and functions: SMACs and the immunological synapse. Semin. Immunol. 15: 295–300
Lee KH, Holdorf AD, Dustin ML, Chan AC, Allen PM and Shaw AS (2002) T cell receptor signaling precedes immunological synapse formation. Science 3: 911–917
Wang D, Matsumoto R, You Y, Che T, Xue-Yan L, Gaffen SL and Lin X (2004) CD3/CD28 costimulation-induced NF-kappaB activation is mediated by recruitment of protein kinase C-theta, Bcl10 and IkappaB kinase beta to the immunological synapse through CARMA1. Mol. Cell. Biol. 24: 164–171
Teixeiro E, Daniels MA, Hausmann B, Schrum AG, Naeher D, Luescher I, Thome M, Bragado R and Palmer E (2004) T cell division and death are segregated by mutation of TCRbeta chain constant domains. Immunity 21: 515–526
Hara H, Bakal C, Wada T, Bouchard D, Rottapel R, Saito T and Penninger JM (2004) The molecular adapter Carma1 controls entry of IkappaB kinase into the central immune synapse. J. Exp. Med. 200: 1167–1177
Weil R, Schwamborn K, Alcover A, Bessia C, Di Bartolo V and Israël A (2003) Induction of the NF-kappaB cascade by recruitment of the scaffold molecule NEMO to the T cell receptor. Immunity 18: 13–26
Lee K-H, Dinner AR, Tu C, Campi G, Raychaudhuri S, Varma R, Sims TN, Burack WR, Wu H, Wang J, Kanagawa O, Markiewicz M, Allen PM, Dustin ML, Chakraborty AK and Shaw AS (2003) The immunological synapse balances T cell receptor signaling and degradation. Science 302: 1218–1222
Wiedemann A, Müller S, Favier S, Penna D, Guiraud M, Delmas C, Champagne E and Valittuti S (2005) T-cell activation is accompanied by an ubiquitination process occurring at the immunological synapse. Immunol. Lett. 98: 57–61
Heissmeyer V, Maciàn F, Im S-H, Varma R, Feske S, Venuprasad K, Gu H, Liu Y-C, Dustin ML and Rao A (2004) Calcineurin imposes T cell unresponsiveness through targeted proteolysis of signaling proteins. Nat. Immunol. 5: 255–265
Bellavia D, Campese AF, Vacca A, Gulino A and Screpanti I (2003) Notch3, another Notch in T cell development. Semin. Immunol. 15: 107–112
Bellavia D, Campese AF, Alesse E, Vacca A, Felli MP, Balestri A and Screpanti I (2000) Constitutive activation of NF-kappaB and T-cell leukemia/lymphoma in Notch3 transgenic mice. EMBO J. 19: 3337–3348
Felli MP, Vacca A, Calce A, Bellavia D, Campese AF, Grillo R, Di Giovine M, Checquolo S, Talora C, Palermo R, Di Mario G, Frati L, Gulino A and Screpanti I (2005) PKCtheta mediates pre-TCR signaling and contributes to Notch3-induced T-cell leukemia. Oncogene 24: 992–1000
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by G Kroemer
Rights and permissions
About this article
Cite this article
Weil, R., Israël, A. Deciphering the pathway from the TCR to NF-κB. Cell Death Differ 13, 826–833 (2006). https://doi.org/10.1038/sj.cdd.4401856
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4401856
Keywords
This article is cited by
-
Probiotic bacteria prevent Salmonella – induced suppression of lymphoproliferation in mice by an immunomodulatory mechanism
BMC Microbiology (2017)
-
Identification of core T cell network based on immunome interactome
BMC Systems Biology (2014)
-
NF-κB pathways in hematological malignancies
Cellular and Molecular Life Sciences (2014)
-
Restrictions to HIV-1 replication in resting CD4+ T lymphocytes
Cell Research (2013)
-
Loss of TCR-beta F1 and/or EZRIN expression is associated with unfavorable prognosis in nodal peripheral T-cell lymphomas
Blood Cancer Journal (2013)