Abstract
CED-9 blocks programmed cell death (apoptosis) in the nematode C. elegans by binding to and neutralizing CED-4, an essential activator of the aspartate-directed cysteine protease (caspase) CED-3. In mammals, the CED-9 homologs Bcl-2 and Bcl-xL also block apoptosis by interfering with the activation of CED-3-like caspases. However, it is unknown whether this occurs by binding to the CED-4 homolog Apaf-1. Whilst two groups previously detected an interaction between Bcl-xL and Apaf-1 in immunoprecipitates,1,2 another group found no interaction between Apaf-1 and any of ten individual members of the Bcl-2 family using the same experimental approach.3 In this study, we aimed to resolve this discrepancy by monitoring the binding of Apaf-1 to three Bcl-2 family members within cells. Using immunofluorescence and Western blot analysis, we show that whilst Apaf-1 is a predominantly cytoplasmic protein, Bcl-2, Bcl-xL and Bax mostly reside on nuclear/ER and mitochondrial membranes. This pattern of localization is maintained when the proteins are co-expressed in both normal and apoptotic cells, suggesting that Bcl-2, Bcl-xL or Bax do not significantly sequester cytoplasmic Apaf-1 to intracellular membranes. In addition, we confirm that Apaf-1 does not interact with Bcl-2 and Bcl-xL in immunoprecipitates. Based on these data, we propose that Apaf-1 is not a direct, physiological target of Bcl-2, Bcl-xL or Bax. Cell Death and Differentiation (2000) 7, 947–954
Similar content being viewed by others
Introduction
Programmed cell death, or apoptosis, is the physiological process that removes superfluous and damaged cells from a multicellular organism.4 Genetic studies in the nematode C. elegans revealed that programmed cell death during development is executed by the aspartate-directed cysteine protease (caspase) CED-3.5 Activation of this protease occurs by binding an adaptor molecule CED-46,7 which oligomerizes and brings into proximity the inactive pro- form of CED-3 enabling it to autoprocess to the active protease.8,9,10 Another protein, CED-9, blocks cell death by forming a complex with CED-4 and attenuating its ability to oligomerize and activate CED-3.6,7,9,10 The ability of CED-9, CED-4 and CED-3 to exist in a multiprotein complex has led to the ‘apoptosome’ model of cell-death regulation.11
Several counterparts of this genetic network have been identified in higher eukaryotes. Up to 14 mammalian CED-3-like caspases are known. Caspases-3 and -7 (and probably also caspase-6) function as executioner or effector caspases because they are activated in all types of apoptosis, their inhibition or chromosomal deletion interfere with apoptosis and/or they cleave most of the cellular substrates implicated in apoptosis.12,13 The other caspases are so called initiator caspases which form a proteolytic cascade on selected signaling pathways (Fas/CD95, cytokine removal, etc.) to cleave and activate the inactive pro-forms of effector caspases.12,13,14 The need for CED-4 homologs in caspase activation was not appreciated until the isolation of the first mammalian homolog Apaf-1.15 Peculiarly, Apaf-1 does not directly activate effector caspases but forms oligomers to facilitate the activation of the initiator caspase-9 which in turn cleaves and activates effector caspases-3 and -7.16,17,18,19 In addition, activation of Apaf-1 requires the binding of the mitochondrial component cytochrome c15,20 which is released into the cytoplasm in response to many apoptotic stimuli.21 Thus, like CED-4, Apaf-1 activates a CED-3-like caspase, but its function depends on mitochondria.
The mammalian homologs of CED-9 are a family of proteins that consists of anti-apoptotic (e.g. Bcl-2) and pro-apoptotic (e.g. Bax) members.22,23,24 In analogy with C. elegans, anti-apoptotic proteins such as Bcl-2 and Bcl-xL might function by binding to CED-4 homologs such as Apaf-1 to prohibit the activation of CED-3-like effector caspases. In support of this notion, Bcl-2 and Bcl-xL have been shown to interfere with the activation of effector caspases,25,26,27 and Bcl-xL has been reported to form a complex with CED-4 upon co-overexpression in mammalian cells.6,28 Moreover, two recent studies have shown that Bcl-xL interacts with Apaf-1 in immunoprecipitates from lysates of cells overexpressing both proteins.1,2 This interaction prevented caspase-9 activation either within a ternary caspase-9/Apaf-1/Bcl-xL complex2 or by disrupting the binding of Apaf-1 to caspase-9.1 Although this finding is in agreement with the nematodal ‘apoptosome’ model, it is inconsistent with the modes of action that have so far been described for Bcl-2 and Bcl-xL. In contrast to CED-9, Bcl-2 and Bcl-xL act by blocking the release of cytochrome c, i.e. at a step occurring before Apaf-1 activation.29,30,31 Although the exact mechanism of this effect is yet unknown, it has been proposed that Bcl-2 and Bcl-xL directly regulate the permeability of the mitochondrial membrane due to their association with this organelle23 and/or their presumed pore forming activity.32,33 Alternatively, they may sequester a cytoplasmic, cytochrome c-release factor such as the Bcl-2 family member BID.34,35 Based on these propositions, Moriishi et al have recently challenged the idea that Apaf-1 and Bcl-xL interact by showing that Bcl-xL or nine other Bcl-2 family members do not co-immunoprecipitate with Apaf-1 from lysates of mammalian cells.3
As Bcl-2 and Bcl-xL are both integral membrane proteins, they have to be solubilized by detergents for immunoprecipitation studies. It has however been reported that detergents can either promote or disrupt interactions between Bcl-2 family members.36,37 We therefore needed to investigate the interaction between Apaf-1 and Bcl-2 family members in an experimental system that does not require detergent extraction. We chose an immunofluorescence analysis on fixed, permeabilized rat embryo fibroblasts overexpressing the proteins and show that three Bcl-2 family members Bcl-2, Bcl-xL, or Bax cannot attract cytoplasmic Apaf-1 to membranes in normal and apoptotic cells. These data raise doubts that Apaf-1 is a direct target of Bcl-2, Bcl-xL or Bax in mammalian cells.
Results
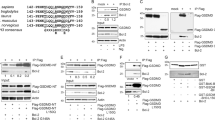
We first studied interactions between Apaf-1 and two anti-apoptotic (Bcl-2 and Bcl-xL) and one pro-apoptotic (Bax) Bcl-2 family members using the previously reported immunoprecipitation approach.1,2,3 For that purpose, N-terminally, Flag-tagged Apaf-1 was transiently co-transfected with either Bcl-2, Bcl-xL or Bax into human embryonic kidney 293 (HEK293) cells. In some cases, the cells were treated for different time periods with apoptotic agents such as the proteasomal inhibitor MG132 to determine whether interactions between Flag-Apaf-1 and Bcl-2 or Flag-Apaf-1 and Bcl-xL are only formed when the latter two proteins act as death suppressors.37 Cell lysates prepared in the presence of the detergent Nonidet P-40 (NP-40) were subjected to anti-Flag, anti-Bcl-2 or anti-Bcl-xL immunoprecipitations, and the immunoprecipitates were Western-blotted for the detection of co-precipitating proteins. As shown in Figures 1 and 3, the proteins were similarly co-expressed so that interactions should have been seen in immunoprecipitates if they indeed occurred. An anti-Flag Western blot showed that Flag-Apaf-1 was present in anti-Flag (Figure 1a, lanes 1 and 3) but not in anti-Bcl-2 (Figure 1a, lane 2) or anti-Bcl-xL (Figure 1a, lane 4) immunoprecipitates of HEK293 cell lysates co-overexpressing Flag-Apaf-1 and Bcl-2 or Flag-Apaf-1 and Bcl-xL, respectively. Similarly, whilst Bcl-2 or Bcl-xL could be specifically immunoprecipitated with anti-Bcl-2 or anti-Bcl-xL antibodies, respectively (Figure 1b, c, lanes 1), they did not co-immunoprecipitate with anti-Flag antibodies from the same cell extracts (Figure 1b, c, lanes 2). This was also the case for extracts prepared from cells exposed to MG132 for 0–24 h (Figure 1, compare −stress and +stress, 24 h). The anti-Flag antibody seemed to co-immunoprecipitate two proteins at 32 and 38 kDa which were detected by the anti-Bcl-xL antibody (Figure 1c). However, upon close examination, the same proteins were also detected by anti-Flag and anti-Bcl-2 antibodies (Figure 1a, lanes 1 and 3 and Figure 1b, lane 2). By contrast, a highly specific anti-Apaf-1 antibody did not co-immunoprecipitate these proteins (Figure 1d, lane 3 in the anti-Bcl-xL panel). This indicates that the 32 and 38 kDa proteins are most likely degradation products of IgG heavy chains of the anti-Flag antibody rather than modified (i.e. phosphorylated) forms of Bcl-xL. The lack of interaction between Flag-Apaf-1 and Bcl-2 or Bcl-xL was not due to the Flag-tag, as the same results were obtained by transfecting non-tagged Apaf-1 and using an anti-Apaf-1 specific antibody (Figure 1d). The latter experiment was also instructive because it did not reveal any interaction between Bcl-2 or Bcl-xL and endogenous Apaf-1 (Figure 1d, lanes 2 and 4 in anti-Apaf-1 panel). Moreover, the failure of these molecules to form complexes was not due to the experimental conditions as anti-Bcl-2 effectively co-immunoprecipitated endogenous Bax, a known physiological partner of Bcl-2.37,38 It was also highly unlikely that the putative Apaf-1/Bcl-2 complex was disrupted by the immunoprecipitation antibodies, because we used both polyclonal (data not shown) and monoclonal (Figure 1) antibodies to Bcl-xL and Bcl-2. We also used two other detergents (digitonin, CHAPS) for cell lysis and immunoprecipitation and still did not find interactions between Apaf-1 and Bcl-2 or Apaf-1 and Bcl-xL (Figure 1e). Unfortunately, we were unable to monitor the interaction between Apaf-1 and Bax as this combination killed most of the co-expressing cells before they could be lysed. Thus, our data confirm the recent report by Moriishi et al3 that Apaf-1 does not form a stable complex with Bcl-2 or Bcl-xL under the conditions used for immunoprecipitations.
Flag-Apaf-1 or Apaf-1 do not interact with Bcl-2 or Bcl-xL in immunoprecipitates. Anti-Flag (a,e), anti-Bcl-2 (b,d), anti-Bcl-xL (c,d), or anti-Apaf-1 (d) Western blots of anti-Flag, anti-Bcl-2, anti-Bcl-xL or anti-Apaf-1 immunoprecipitates (IP) from 0.2% NP-40 (a–d), 0.2% CHAPS (e) or 0.5% digitonin (e) lysates of HEK293 cells transfected with Flag-Apaf-1 alone or co-transfected with Flag-Apaf-1 and human Bcl-2 (hBcl-2), Flag-Apaf-1 and mouse Bcl-2 (mBcl-2), Flag-Apaf-1 and human Bcl-xL (hBcl-xL), Apaf-1 and human Bcl-2, or Apaf-1 and human Bcl-xL, treated (+stress) or not (−stress) with 1 μM of the proteasome inhibitor MG132 for 24 h. Note that exogenous Flag-Apaf-1 is present in anti-Flag (a, lanes 1 and 3, e, lanes 1) but not in anti-Bcl-2 (a, lanes 2, e, lanes 3) or anti-Bcl-xL (a, lanes 4, e, lanes 2) IPs from co-transfected cells. Similarly, Apaf-1 is present in anti-Apaf-1 (d, lanes 1 and 3, left panel), but not in anti-Bcl-2 (d, lane 2, left panel) or anti-Bcl-xL (d, lane 4, left panel) IPs. By contrast, exogenous Bcl-2 is present in anti-Bcl-2 (b, lanes 1, d, lane 2, middle panel) but not in anti-Flag (b, lanes 2) or anti-Apaf-1 (d, lane 1, middle panel) IPs and exogenous Bcl-xL is present in anti-Bcl-xL (c, lanes 1, d, lane 4, right panel) but not in anti-Flag (c, lanes 2) or anti-Apaf-1 (d, lane 3, right panel) IPs from co-transfected cells. The two bands at 32 and 38 kDa detected in anti-Flag IPs (c, lanes 2) correspond to cross-reactive proteins as they are not detected in anti-Apaf-1 IPs (d, lane 3, right panel). Sometimes the secondary antibody reacted with the heavy IgG (ca. 50 kDa) of the primary antibody
Apaf-1 is predominantly cytoplasmic while Bcl-2 and Bcl-xL are mainly found in nuclear and membrane fractions. (a) Anti-Flag, anti-Bcl-2 or anti-Bcl-xL Western blots of cytosolic, nuclear and membrane fractions of HEK293 cells transfected with Flag-Apaf-1 or co-transfected with Flag-Apaf-1 and hBcl-2 or Flag-Apaf-1 and hBcl-xL. (b) Anti-Apaf-1, anti-Bcl-2 or anti-Bcl-xL Western blots of cytosolic, nuclear and membrane fractions of HEK293 cells transfected with Apaf-1, hBcl-2 or hBcl-xL or co-transfected with Apaf-1 and hBcl-2 or Apaf-1 and hBcl-xL
Although we tested different detergents (NP-40, CHAPS, digitonin) for our in vitro experiments, it was still possible that Bcl-2 and Bcl-xL interacted with Apaf-1 in cells, but that this interaction was disrupted during cell lysis and/or immunoprecipitation. To resolve this issue, we performed an immunofluorescence analysis on rat embryo fibroblasts (R6), Hela, HEK293 and SW480 human colon carcinoma cell lines which had previously been co-transfected with Flag-Apaf-1 and Bcl-2, Flag-Apaf-1 and Bcl-xL or Flag-Apaf-1 and Bax. Here we show the results in R6 cells but similar results were obtained in the other cell lines. After transient transfection, Flag-Apaf-1 displayed a diffuse immunostaining in the cytoplasm with both anti-Flag and anti-Apaf-1 antibodies (Figure 2a, Flag-Apaf-1). This staining was specific as non-transfected cells revealed only a low background staining with both antibodies (see neighboring cell in Figure 2a, anti-Apaf-1). The nuclear staining of Flag-Apaf-1 was probably due to the non-confocal type of immunofluorescence method used here as no Flag-Apaf-1 was detected in nuclear fractions isolated from Flag-Apaf-1-transfected cells (Figure 3a, nuclei). In contrast to the diffuse immunostaining of Apaf-1, Bcl-xL was observed on elongated, ‘spaghetti-like’ structures that co-localized with the mitochondrial marker cytochrome c oxidase (COX) (Figure 2a, Bcl-xL, and data not shown). Bax was also clearly mitochondrial, but in a punctated pattern, and the cells containing high levels of Bax were dying by apoptosis (Figure 2a, Bax, and ref39). Finally, Bcl-2 showed a broad subcellular localization pattern that included the nuclear envelope and the endoplasmic reticulum as previously reported (Figure 2a, Bcl-2).40 Thus, if Flag-Apaf-1 interacted with Bcl-2 or Bcl-xL upon co-overexpression, it should migrate to their sites of localization. However, despite effective and equal co-overexpression with Bcl-2, Bcl-xL or Bax, Flag-Apaf-1 continued to display a predominantly cytoplasmic localization (Figure 2b). The same result was seen when the cells were stressed with MG132 or other apoptotic stimuli for different time periods (Figure 2c, 1 μM MG132 for 1 h is shown here). Thus, Apaf-1 does not seem to be attracted to intracellular sites where Bcl-2, Bcl-xL or Bax reside.
Bcl-2, Bcl-xL or Bax cannot attract cytoplasmic Flag-Apaf-1 to intracellular membranes in both normal and apoptotically stressed cells. (a) Anti-Flag or anti-Apaf-1 (fluorescein), and anti-Bcl-2, anti-Bcl-xL or anti-Bax (all Texas Red) immunofluorescence analysis on rat embryo fibroblasts (R6) transfected with Flag-Apaf-1, hBcl-2, hBcl-xL or hBax for 15 h, respectively. (b) Anti-Apaf-1 (fluorescein) and anti-Bcl-2, anti-Bcl-xL or anti-Bax (all Texas Red) immunofluorescence analysis on R6 cells co-transfected with Flag-Apaf-1 and hBcl-2 or Flag-Apaf-1 and hBcl-xL or Flag-Apaf-1 and hBax for 15 h, respectively. (c) Immunofluorescence analysis of co-transfected R6 cells as in (b) but after treating the cells with 1 μM MG132 for 1 h (out of 15 h total transfection time). Note that Flag-Apaf-1 and Apaf-1 show a diffuse, cytoplasmic/nuclear staining irrespective of whether it is co-transfected with Bcl-2 (nuclear envelope/endoplasmic reticulum), Bcl-xL (mitochondria, elongated) or Bax (mitochondria, punctuated) and/or whether the cells are stressed with the apoptotic agent MG132. The cells expressing Bax undergo apoptosis (nuclear fragmentation) (data not shown)
To confirm that Apaf-1 was mostly cytoplasmic and Bcl-2 or Bcl-xL were mostly membrane-bound even in co-transfected cells, we performed Western blots of cytosolic, membrane and nuclear fractions of HEK293 cells either transfected with Flag-Apaf-1, Apaf-1, Bcl-2 or Bcl-xL alone or co-transfected with Flag-Apaf-1 (or Apaf-1) and Bcl-2 or Flag-Apaf-1 (or Apaf-1) and Bcl-xL. Although some Flag-Apaf-1 and Apaf-1 were detected in the membrane fraction, most of these proteins resided in the cytosol, irrespective of the co-presence of overexpressed Bcl-2 or Bcl-xL (Figure 3a, b). By contrast, both Bcl-2 and Bcl-xL localized to membranes or nuclei, and this distribution did not change in the presence of overexpressed Flag-Apaf-1 (Figure 3a) or Apaf-1 (Figure 3b). Thus, Apaf-1 has a cytoplasmic localization distinct from Bcl-2 or Bcl-xL.
Discussion
Despite intensive research, it has remained unknown how anti-apoptotic Bcl-2 family members prevent caspase activation. Two modes of action have been proposed, one which indirectly and another which directly affects Apaf-1 function. The indirect model suggests that Bcl-2 and/or Bcl-xL act on the outer mitochondrial membrane to prevent the release of cytochrome c,29,30,31 a necessary co-factor for Apaf-1 oligomerization and the subsequent activation of caspase-9 and -3.15,16,17,20 The direct model suggests a physical interaction between Bcl-2 and Apaf-1 or Bcl-xL and Apaf-1. Heterologous overexpressions of the C. elegans death regulators in mammalian cells have shown that CED-9, CED-4 and CED-3 exist in a multiprotein complex, dubbed the apoptosome, in which CED-9 inhibits apoptosis by binding to CED-4, thereby preventing it from activating CED-3.6,7,9,10,11 Interestingly, such interactions have not only been detected by co-immunoprecipitation but also using immunofluorescence analysis where soluble CED-4 was sequestered to the perinuclear region by CED-9.28 The latter study showed that it should be possible to detect a similar attraction between Apaf-1 and Bcl-2 or Bcl-xL if the analogous mammalian apoptosome indeed consisted of these factors.
Our data disfavor the notion that Apaf-1 forms a mammalian apoptosome with Bcl-2 or Bcl-xL. Why are our results in conflict with two previous studies that have reported direct interactions between Bcl-xL and Apaf-1?1,2 Both studies used immunoprecipitation approaches to determine protein-protein interactions. Although Moriishi et al3 and we have used immunoprecipitation protocols that closely replicated those reported1,2 it may be difficult to reach the exact same experimental conditions for reproducing the results. For example, we noticed that, when in abundance, Apaf-1, Bcl-2 and Bcl-xL all bind non-specifically to Sepharose beads (data not shown), and thus part of the presumed Apaf-1/Bcl-2 or Apaf-1/Bcl-xL complexes may originate from such artefacts. In addition, there remains the possibility that the quality of the detergents influences complex formations during cell lysis and immunoprecipitation. Our use of immunofluorescence to study the interaction between Apaf-1 and Bcl-2 family members within cells circumvents these in vitro problems. In several different cell types co-transfected with Flag-Apaf-1, Apaf-1, Bcl-2, Bcl-xL or Bax and exposed to different apoptotic stimuli for various time periods, we have not seen any evidence that the diffusely stained, cytoplasmic Apaf-1 is sequestered to nuclear or mitochondrial membranes in a similar fashion that CED-9 sequesters CED-4.28 Is it possible that Apaf-1 was recruited to Bcl-2 or Bcl-xL at levels undetectable by immunofluorescence? Although such a possibility cannot be entirely excluded, we think that we should have seen a major sequestration by this technique as the apoptosome model in C. elegans accounts for a tight complex formation between CED-9 and CED-4 in healthy cells.28,41 In addition, Western blot analysis of subcellular fractions of HEK293 cells co-transfected with Flag-Apaf-1/Bcl-2 or Flag-Apaf-1/Bcl-xL revealed that both Apaf-1 and Flag-Apaf-1 were predominantly present in the cytosol rather than in membrane fractions. Thus, instead of being sequestered to membrane-associated Bcl-2 or Bcl-xL, Apaf-1 seems to constitutively reside in the cytoplasm to await the release of cytochrome c for the activation of caspase-9. A novel CED-4 homolog, named Nod1/CARD442,43 has recently been identified, and it will be interesting to determine whether it fulfills the criteria to form an apoptosome with Bcl-2 or Bcl-xL. Based on the findings that Bcl-2 and Bcl-xL prevent the release of cytochrome c, it is expected that these proteins bind to and inhibit a CED-4 homolog that functions upstream of mitochondria to initiate the release of cytochrome c and other apoptogenic factors.
Materials and Methods
Subcellular fractionation, immunoprecipitations and Western blot analysis
HEK293 cells were co-transfected with 5 μg of each human Flag-Apaf-1/pcDNA3 (kindly provided by V Dixit), human Apaf-1/pcDNA3.1(−) (kindly provided by X Wang), human Bcl-2/pcDNA3, mouse Bcl-2/pcDNA3 or human Bcl-xL/pcDNA3 (kindly provided by G Nuñez) using 25 μl of Superfect (Qiagen) as described by the manufacturer. In some cases, the cells were treated with 1 μM of MG132 (Alexis) for 0–24 h. The cells were lysed in 0.2% NP-40, 0.2% CHAPS or 0.5% digitonin and subjected to immunoprecipitation using 3 μl of mouse monoclonal anti-hBcl-2/100 (Neomarker), 5 μl of rabbit polyclonal anti-m/rBcl-2/27-6,37 3 μl of mouse monoclonal anti-Bcl-xL/2H12 (Zymed), 2.5 μl of mouse monoclonal anti-Flag/M5 (Sigma) or 5 μl of rabbit polyclona anti-Apaf-1/IP14 (Zymed) and 50 μl of 50% (v/v) protein G-Sepharose (PharmaciaAmersham) or protein A-Sepharose (Sigma) as previously described.37 Immunocomplexes were subjected to Western blot analysis using either anti-hBcl-2/100 at a titer of 1 : 200, anti-Bcl-xL/2H12 at a titer of 1 : 1000, anti-Flag/M5 at a titer of 1 : 1000 or anti-Apaf-I/IP14 at a titer of 1 : 1000. Secondary antibodies were Fcγ-specific, peroxidase-coupled goat anti-rabbit or anti-mouse antibodies (Jackson ImmunoResearch Laboratories), and the detection system was enhanced chemiluminescence (ECL, AmershamPharmacia). For subcellular fractionation analysis, the co-transfected HEK293 cells were lysed in buffer A/sucrose (20 mM HEPES, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EGTA, 1 mM EDTA, 1 mM DTT, 0.25 M sucrose, 10 μg/ml leupeptin, 2 μg/ml aprotinin, 0.4 mM Pefabloc, 1 μg/ml pepstatin) using a tight fitting Dounce homogenizer. The lysates were centrifuged at 750×g to obtain a nuclear pellet. The postnuclear supernatant was further centrifuged at 100 000×g to obtain a membrane pellet and the cytosol. Thirty μg of each fraction was loaded on polyacrylamide gels for Western blot analysis.
Immunofluorescence analysis
R6 cells were transfected with 0.8 μg of each Flag-hApaf-1/pcDNA3, hBcl-2/pcDNA3, hBcl-xL/pcDNA3 or hBax/pcDNA3 in 2.4 μl (single transfections) or 4.8 μl (double transfections) Superfect as described by the manufacturer (Qiagen). The cells were fixed in 4% paraformaldehyde, and permeabilized with 0.05% saponin and acetone as previously described.39 The cells were treated with mouse monoclonal anti-Flag (1 : 100), rabbit polyclonal anti-Apaf-1/IP14 (1 : 50), mouse monoclonal anti-hBcl-2/100 (1 : 100), mouse monoclonal anti-Bcl-xL/2H12 (1 : 100) or mouse monoclonal anti-hBax/2D2 (1 : 100) (Zymed) for 1 h followed by incubations with fluorescein- and Texas Red-conjugated goat anti-rabbit and anti-mouse secondary antibodies (Jackson ImmunoResearch Laboratories). After postfixation in 4% paraformaldehyde, the antifading agent Slowfade (Molecular Probes) was added, and the cells were viewed under a regular Zeiss Axiovert fluorescence microscope at a magnification of 1000×. Pictures were taken with a Contax 167 MT camera.
Note added in proof
While this paper was under review, Hausmann et al44 published that Apaf-1 does not colocalize with Bcl-2 or Bcl-xL when analyzed by immunofluorescence and immunogold electron microscopy. Moreover, Newmeyer et al45 and Haraguchi et al46 used in vitro assays and cells deficient in Apaf-1, respectively, to show that Bcl-2 and Bcl-xL do not require Apaf-1 for their caspase-inhibiting and death protective function.
Abbreviations
- HEK:
-
human embryonic kidney
- R6:
-
rat 6 embryo fibroblasts
- CED:
-
product of cell-death-abnormal gene
- caspase:
-
cysteinyl aspartate-specific proteinase
- Apaf-1:
-
apoptotic protease activating factor-1
- BH:
-
Bcl-2 homology domain
- COX:
-
cytochrome c oxidase
- NP-40:
-
Nonidet P-40
- CHAPS:
-
3-[(3-cholamidopropyl)dimethylammonio]-1-propane-sulfonate
- ER:
-
endoplasmic reticulum
- IP:
-
immunoprecipitation
References
Hu Y, Benedict MA, Wu D, Inohara N and Nuñez G . 1998 Bcl-xL interacts with Apaf-1 and inhibits Apaf-1-dependent caspase-9 activation. Proc. Natl. Acad. Sci. U.S.A. 95: 4386–4391
Pan G, O'Rourke K and Dixit VM . 1998 Caspase-9, Bcl-xL, and Apaf-1 form a ternary complex. J. Biol. Chem. 273: 5841–5845
Moriishi K, Huang DC, Cory S and Adams JM . 1999 Bcl-2 family members do not inhibit apoptosis by binding the caspase activator Apaf-1. Proc. Natl. Acad. Sci. U.S.A. 96: 9683–9688
Vaux DL and Korsmeyer SJ . 1999 Cell death in development. Cell 96: 245–254
Shaham S and Horvitz HR . 1996 Developing Caenorhabditis elegans neurons may contain both cell-death protective and killer activities. Genes Dev. 10: 578–591
Chinnaiyan AM, O'Rourke K, Lane BR and Dixit VM . 1997 Interaction of CED-4 with CED-3 and CED-9: a molecular framework for cell death. Science 275: 1122–1126
Wu D, Wallen HD, Inohara N and Nuñez G . 1997 Interaction and regulation of the C. elegans death protease CED-3 by CED-4 and CED-9. J. Biol. Chem. 272: 21449–21454
Chinnaiyan AM, Chaudhary D, O'Rourke K, Koonin EV and Dixit VM . 1997 Role of CED-4 in the activation of CED-3. Nature 388: 728–729
Seshagiri S and Miller LK . 1997 Caenorhabditis elegans CED-4 stimulates CED-3 processing and CED-3-induced apoptosis. Curr. Biol. 7: 455–460
Yang X, Chang HY and Baltimore D . 1998 Essential role of CED-4 oligomerization in CED-3 activation and apoptosis. Science 281: 1355–1357
Hengartner MO . 1997 CED-4 is a stranger no more. Nature 388: 714–715
Earnshaw WC, Martins LM and Kaufmann SH . 1999 Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu. Rev. Biochem. 68: 383–424
Nicholson DW . 1999 Caspase structure, proteolytic substrates, and function during apoptotic cell death. Cell Death Differ. 6: 1028–1042
Kumar S . 1999 Mechanisms mediating caspase activation in cell death. Cell Death Differ. 6: 1060–1066
Zou H, Henzel WJ, Liu X, Lutschg A and Wang X . 1997 Apaf-1, a human protein homologous to C. elegans CED-4, participates in cytochrome c-dependent activation of caspases. Cell 90: 405–413
Li P, Nijhawan D, Budihardjo I, Srinivasula SM, Ahmad M, Alnemri ES and Wang X . 1997 Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 91: 479–489
Srinivasula SM, Ahmad M, Fernandes-Alnemri T and Alnemri ES . 1998 Autoactivation of procaspase-9 by Apaf-1-mediated oligomerization. Mol. Cell 1: 949–957
Cain K, Brown DG, Langlais C and Cohen GM . 1999 Caspase activation involves the formation of the aposome, a large (∼700 kDa) caspase-activating complex. J. Biol. Chem. 274: 22686–22692
Cecconi F . 1999 Apaf-1 and the apoptotic machinery. Cell Death Differ. 6: 1087–1098
Hu Y, Benedict MA, Ding L and Nuñez G . 1999 Role of cytochrome c and dATP/ATP hydrolysis in Apaf-1-mediated caspase-9 activation and apoptosis. EMBO J. 18: 3586–3595
Green DR and Reed JC . 1998 Mitochondria and apoptosis. Science 281: 1309–1312
Adams JA and Cory S . 1998 The Bcl-2 protein family: arbiters of cell survival. Science 281: 1322–1326
Reed J . 1998 Bcl-2 family proteins. Oncogene 17: 3225–3236
Kelekar A and Thompson CB . 1998 Bcl-2 family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol. 8: 324–330
Monney L, Otter I, Olivier R, Ravn U, Mirzasaleh H, Fellay I, Poirier GG and Borner C . 1996 Bcl-2 overexpression blocks activation of the death protease CPP32/Yama/Apopain. Biochem. Biophys. Res. Commun. 221: 340–345
Chinnaiyan AM, Orth K, O'Rourke K, Duan H, Poirier GG and Dixit VM . 1996 Molecular Ordering of the cell death pathway: Bcl-2 and Bcl-xL function upstream of the CED-3-like apoptotic proteases. J. Biol. Chem. 271: 4573–4576
Boulakia CA, Chen G, Ng FWH, Teodore JG, Branton PE, Nicholson DW, Poirier GG and Shore GC . 1996 Bcl-2 and adenovirus E1B 19 kDA protein prevent E1A-induced processing of CPP32 and cleavage of poly(ADP-ribose) polymeras. Oncogene 12: 529–535
Wu D, Wallen HD and Nuñez G . 1997 Interaction and regulation of subcellular localization of CED-4 by CED-9. Science 275: 1126–1129
Kluck RM, Bossy-Wetzel E, Green DR and Newmeyer DD . 1997 The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275: 1132–1136
Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng T-I, Jones DP and Wang X . 1997 Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275: 1129–1132
Kharbanda S, Pandey P, Schofield L, Israels S, Roncinske R, Yoshida K, Bharti A, Yuan Z-M, Saxena S, Weichselbaum R, Nalin C and Kufe D . 1997 Role for Bcl-xL as an inhibitor of cytosolic cytochrome c accumulation in DNA damage-induced apoptosis. Proc. Natl. Acad. Sci. U.S.A. 94: 6939–6942
Minn AJ, Vélez P, Schendel SL, Liang H, Muchmore SW, Fesik SW, Fill M and Thompson CB . 1997 Bcl-xL forms an ion channel in synthetic lipid membranes. Nature 385: 353–357
Schendel SL, Xie Z, Montal MO, Matsuyama S, Montal M and Reed JC . 1997 Channel formation by antiapoptotic protein Bcl-2. Proc. Natl. Acad. Sci. U.S.A. 94: 5113–5118
Luo X, Budihardjo I, Zou H, Slaughter C and Wang X . 1998 Bid, a Bcl-2 interacting protein, mediates cytochrome c release from mitochondria in response to activation of cell surface death receptors. Cell 94: 481–490
Li H, Zhu H, Xu C-J and Yuan J . 1998 Cleavage of BID by caspase 8 mediates the mitochondrial damage in the Fas pathway to apoptosis. Cell 94: 491–501
Hsu Y-T and Youle RJ . 1997 Nonionic detergents induce dimerization among members of the Bcl-2 family. J. Biol. Chem. 272: 13829–13834
Otter I, Conus S, Ravn U, Rager M, Olivier R, Monney L, Fabbro D and Borner C . 1998 The binding properties and biological activities of Bcl-2 and Bax in cells exposed to apoptotic stimuli. J. Biol. Chem. 273: 6110–6120
Oltvai ZN, Milliman CL and Korsmeyer SJ . 1993 Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programed cell death. Cell 74: 609–619
Rossé T, Olivier R, Monney L, Rager M, Conus S, Fellay I, Jansen B and Borner C . 1998 Bcl-2 prolongs cell survival after Bax-induced release of cytochrome c. Nature 391: 496–499
Givol I, Tsarfaty I, Resau J, Rulong S, Pinto da Silva P, Nasioulas G, DuHadaway J, Hughes SH and Ewert DL . 1994 Bcl-2 expressed using a retroviral vector is localized primarily in the nuclear membrane and the endoplasmic reticulum of chicken embryo fibroblasts. Cell Growth Differ. 5: 419–424
Spector MS, Desnoyers S, Hoeppner DJ and Hengartner MO . 1997 Interaction between the C. elegans cell-death regulators CED-9 and CED-4. Nature 385: 653–656
Inohara N, Koseki T, del Peso L, Hu Y, Yee C, Chen S, Carrio R, Merino J, Liu D, Ni J and Nuñez G . 1999 Nod1, an Apaf-1-like activator of caspase-9 and nuclear factor-kB. J. Biol. Chem. 274: 14560–14567
Bertin J, Nir W-J, Fischer CM, Tayber OV, Errada PR, Grant JR, Keilty JJ, Gosselin ML, Robison KE, Wong GHW, Glucksmann AM and DiStefano PS . 1999 Human CARD4 protein is a novel CED-4/Apaf-1 cell death family member that activates NF-kB. J. Biol. Chem. 274: 12955–12958
Hausmann G, O'Reilly LA, van Driel R, Beaumont JG, Strasser A, Adams JM and Huang DCS . 2000 Pro-apoptotic apoptosis protease-activating factor 1 (Apaf-1) has a cytoplasmic localization distinct from Bcl-2 and Bcl-xL . J Cell Biol. 149: 623–633
Newmeyer DD, Bossy-Wetzel E, Kluck RM, Wolf BB, Beere HM and Green DR . 2000 Bcl-xL does not inhibit the function of apaf-1. Cell Death Differ 7: 402–407
Haraguchi M, Torii S, Matsuzawa S-I, Xie Z, Kitada S, Krajewski S, Yoshida H, Mak TW and Reed JC . 2000 Apoptotic protease activating factor (Apaf-1)-independent cell death suppression by Bcl-2. J. Exp. Med. 191: 1709–1720
Acknowledgements
We thank Xiaodong Wang for the Apaf-1 cDNA, Vishva Dixit for the Flag-Apaf-1 cDNA, and Gabriel Nuñez for the Bcl-xL cDNA. We are grateful to Anna Schinzel, Myriam Tapernoux and Javier Sanz for critically reading the manuscript. This work was supported by the Swiss National Science Foundation (#31-46930.96). C Borner is the recipient of a young investigator fellowship (START) by the Swiss National Science Foundation (#31-34600.92).
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by D Vaux
Rights and permissions
About this article
Cite this article
Conus, S., Rossé, T. & Borner, C. Failure of Bcl-2 family members to interact with Apaf-1 in normal and apoptotic cells. Cell Death Differ 7, 947–954 (2000). https://doi.org/10.1038/sj.cdd.4400729
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4400729
Keywords
This article is cited by
-
Living with death: the evolution of the mitochondrial pathway of apoptosis in animals
Cell Death & Differentiation (2008)
-
Bcl-2 protein family: Implications in vascular apoptosis and atherosclerosis
Apoptosis (2006)
-
Nuclear–cytoplasmic translocation of BARD1 is linked to its apoptotic activity
Oncogene (2004)
-
Bcl-xES, a BH4- and BH2-containing antiapoptotic protein, delays Bax oligomer formation and binds Apaf-1, blocking procaspase-9 activation
Oncogene (2004)
-
Apaf1 is no longer single
Cell Death & Differentiation (2001)