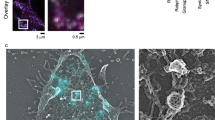
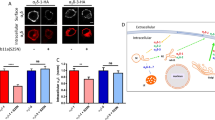
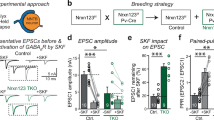
Abstract
The Rab family of low-molecular-mass GTP-binding proteins are thought to guide membrane fusion between a transport vesicle and the target membrane, and to determine the specificity of docking1,2,3. The docking and fusion of vesicles is, however, a complex multistep reaction, and the precise point at which Rab proteins act in these sequential processes is unknown. In brain, the Rab protein Rab3A is specific to synaptic vesicles, whose exocytosis can be monitored with submillisecond resolution by following synaptic transmission. We have now determined the precise point at which Rab3A acts in the sequence of synaptic vesicle docking and fusion by using electrophysiological analysis of neurotransmitter release in Rab3A-deficient mice. Unexpectedly, the size of the readily releasable pool of vesicles is normal, whereas Ca2+-triggered fusion is altered in the absence of Rab3A in that a more-than-usual number of exocytic events occur within a brief time after arrival of the nerve impulse.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Südhof, T. C. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature 375, 645–653 (1995).
Scheller, R. H. Membrane trafficking in the presynaptic nerve terminal. Neuron 14, 893–897 (1995).
Pfeffer, S. R. Rab GTPases: master regulators of membrane trafficking. Curr. Opin. Cell Biol. 6, 522–526 (1994).
Katz, B. The Release of Neural Transmitter Substances(Liverpool University Press, Liverpool, (1969)).
Hubbard, J. I. Repetitive stimulation at the mammalian neuromuscular junction, and the mobilization of transmitter. J. Physiol. (Lond.) 169, 641–662 (1963).
Geppert, M. et al. The role of rab3A in neurotransmitter release. Nature 369, 493–497 (1994).
Stevens, C. F. & Tsujimoto, T. Extimates for the pool size of releasable quanta at a single central synapse and for the time required to refill the pool. Proc. Natl Acad. Sci. USA 92, 846–849 (1995).
Geppert, M. et al. Synaptotagmin I: a major Ca2+sensor for transmitter release at a central synapse. Cell 79, 717–727 (1994).
Rosenmund, C. & Stevens, C. F. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron 16, 1197–1207 (1996).
Mallart, A. & Martin, A. The relation between quantal content and facilitation at the neuromuscular junction of the frog. R. J. Physiol., (Lond.) 196, 593–604 (1968).
Kamiya, H. & Zucker, R. S. Residual Ca2+and short-term synaptic plasticity. Nature 371, 603–606 (1994).
Huettner, J. E. & Bean, B. P. Block of N-methyl-D-aspartate-activated current by the anticonvulsant MK-801: selective binding to open channels. Proc. Natl Acad. Sci. USA 85, 1307–1311 (1988).
Rosenmund, C., Clements, J. D. & Westbrook, G. L. Non-uniform probability of glutamate release at a hippocampal synapse. Science 262, 754–757 (1993).
Hessler, N. A., Shirke, A. M. & Manilow, R. The probability of transmitter release at a mammalian central synapse. Nature 366, 569–572 (1993).
Huang, E. & Stevens, C. F. Estimating the distribution of synaptic reliabilities. J. Neurophysiol.(submitted).
Clements, J. D., Lester, R. A. J., >Tong, G., Jahr, C. E. & Westbrook, G. L. The time course of glutamate in the synaptic cleft. Science 258, 1498–1501 (1992).
Perkel, D. J. & Nicoll, R. A. Evidence for all-or-none regulation of neurotransmitter release: implications for long-term potentiation. J. Physiol., (Lond.) 471, 481–500 (1993).
Olverman, H. J., Jones, A. W., Mewett, K. N. & Watkins, J. C. Structure/activity relations of N-methyl-D-aspartate receptor ligands as studied by their inhibition of [3H]D-2-amino-5-phosphonopentanoic acid binding in rat brain membranes. Neuroscience 26, 17–31 (1988).
Holz, R. W., Brondyk, W. H., Senter, R. A., Kuizon, L. & Macara, I. G. Evidence for the involvement of Rab3A in Ca2+-dependent exocytosis from adrenal chromaffin cells. J. Biol. Chem. 269, 10229–10234 (1994).
Johannes, L. et al. The GTPase rab3A negatively controls calcium-dependent exocytosis in neuroendocrine cells. EMBO J. 13, 2029–2037 (1994).
Stahl, B., Chou, J. H., Li, C., Südhof, T. C. & Jahn, R. Rab3 reversibly recruits rabphilin to synaptic vesicles by a mechanism analogous to raf recruitment by ras. EMBO J. 15, 1799–1809 (1996).
Mallart, A. & Martin, A. R. An analysis of facilitation of transmitter release at the neuromuscular junction of the frog. J. Physiol. (Lond.) 193, 593–604 (1967).
Manabe, T., Wyllie, D. J. A., Perkel, D. J. & Nicoll, R. A. Modulation of synaptic transmission and long-term potentiation: effects of paired pulse facilitation and EPSC variance in the CA1 region of the hippocampus. J. Neurophysiol. 70, 1451–1459 (1993).
Schulz, P. E., Cook, E. P. & Johnston, D. Changes in paired-pulse facilitation suggest presynaptic involvement in long-term potentiation. J. Neurosci. 14, 5325–5337 (1994).
Raastad, M., Storm, J. F. & Andersen, P. Putative single quantum and single fibre excitatory postsynaptic currents show similar amplitude range and variability in rat hippocampal slices. Eur. J. Neurosci. 4, 113–117 (1992).
Allen, C. & Stevens, C. F. An evaluation of causes for unreliability of synaptic transmission. Proc. Natl Acad. Sci. USA 91, 10380–10383 (1994).
Stevens, C. F. & Wang, Y. Changes in reliability of synaptic function as a mechanism for plasticity. Nature 371, 704–707 (1994).
Stevens, C. F. & Wang, Y. Facilitation and depression at single central synapses. Neuron 14, 795–802 (1995).
Triller, A. & Korn, H. Transmission at a central inhibitory synapse. III. Ultrastructure of physiologically identified and stained terminals. J. Neurophysiol. 48, 708–736 (1982).
Rybin, V. et al. GTPase activity of Rab5 acts as a timer for endocytic membrane fusion. Nature 383, 266–269 (1996).
Acknowledgements
The authors are listed in alphabetical order. We thank J. Wesseling for discussions, and C. Boyer for help in the culture preparation. This work was supported by the Howard Hughes Medical Institute (C.F.S. and T.C.S.), the NIH (C.F.S.) and the National Alliance for Research on Schizophrenia and Depression (Y.G.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Geppert, M., Goda, Y., Stevens, C. et al. The small GTP-binding protein Rab3A regulates a late step in synaptic vesicle fusion. Nature 387, 810–814 (1997). https://doi.org/10.1038/42954
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/42954
This article is cited by
-
Integrating intracellular nanovesicles into integrin trafficking pathways and beyond
Cellular and Molecular Life Sciences (2022)
-
miR-142a-3p promotes the proliferation of porcine hemagglutinating encephalomyelitis virus by targeting Rab3a
Archives of Virology (2020)
-
Synaptic loss in schizophrenia: a meta-analysis and systematic review of synaptic protein and mRNA measures
Molecular Psychiatry (2019)
-
Chronic treatment with fluoride affects the jejunum: insights from proteomics and enteric innervation analysis
Scientific Reports (2018)
-
PAR3–PAR6–atypical PKC polarity complex proteins in neuronal polarization
Cellular and Molecular Life Sciences (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.