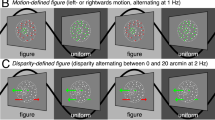
Abstract
Edges are important in the interpretation of the retinal image. Although luminance edges have been studied extensively, much less is known about how or where the primate visual system detects boundaries defined by differences in surface properties such as texture, motion or binocular disparity. Here we use functional magnetic resonance imaging (fMRI) to localize human visual cortical activity related to the processing of one such higher-order edge type: motion boundaries. We describe a robust fMRI signal that is selective for motion segmentation. This boundary-specific signal is present, and retinotopically organized, within early visual areas, beginning in the primary visual cortex (area V1). Surprisingly, it is largely absent from the motion-selective area MT/V5 and far extrastriate visual areas. Changes in the surface velocity defining the motion boundaries affect the strength of the fMRI signal. In parallel psychophysical experiments, the perceptual salience of the boundaries shows a similar dependence on surface velocity. These results demonstrate that information for segmenting scenes by relative motion is represented as early as V1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Braddick, O. Ashort-range process in apparent motion. Vision Res. 14, 519–527 (1974).
Regan, D., Giaschi, D., Sharpe, J. A. & Hong, X. H. Visual processing of motion-defined form: selective failure in patients with parietotemporal lesions. J. Neurosci. 12, 2198–2210 (1992).
Dick, M., Ullman, S. & Sagi, D. Parallel and serial processes in motion detection. Science 237, 400–402 (1987).
Lamme, V. A., van Dijk, B. W. & Spekreijse, H. Contour from motion processing occurs in primary visual cortex. Nature 363, 541–543 (1993).
Vaina, L. M., Grzywacz, N. M. & Kikinis, R. Segregation of computations underlying perception of motion discontinuity and coherence. Neuroreport 5, 2289–2294 (1994).
Murakami, I. & Shimojo, S. Modulation of motion aftereffect by surround motion and its dependence on stimulus size and eccentricity. Vision Res. 35, 1835–1844 (1995).
Nakayama, K. & Loomis, J. M. Optimal velocity patterns, velocity-sensitive neurons, and space perception: a hypothesis. Perception 3, 63–80 (1974).
Patzwahl, D. R., Zanker, J. M. & Altenmuller, E. O. Cortical potentials reflecting motion processing in humans. Vis. Neurosci. 11, 1135–1147 (1994).
Orban, G. A. et al. Amotion area in human visual cortex. Proc. Natl Acad. Sci. USA 92, 993–997 (1995).
Lagae, L., Gulyas, S., Raiguel, S. & Orban, G. A. Laminar analysis of motion information processing in macaque V5. Brain Res. 496, 361–367 (1989).
Engel, S. A. et al. fMRI of human visual cortex. Nature 369, 525 (1994).
Sereno, M. I. et al. Borders of multiple visual areas in humans revealed by functional magnetic resonance imaging. Science 268, 889–893 (1995).
DeYoe, E. A. et al. Mapping striate and extrastriate visual areas in human cerebral cortex. Proc. Natl Acad. Sci. USA 93, 2382–2386 (1996).
Tootell, R. B. H. et al. Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J. Neurosci. 15, 3215–3230 (1995).
Reppas, J. B., Dale, A. M., Sereno, M. I. & Tootell, R. B. H. La vision: une perception subjective. La Recherche 289, 52–56 (1996).
Maunsell, J. H. R. & van Essen, D. C. Functional properties of neurons in middle temporal visual area of the macaque monkey. I. Selectivity for stimulus direction, speed, and orientation. J. Neurophysiol. 49, 1127–1147 (1983).
Braddick, O. Segmentation versus integration in visual motion processing. Trends Neurosci. 16, 263–268 (1993).
Snowden, R. J., Treue, S., Erickson, R. E. & Andersen, R. A. The response of area MT and V1 neurons to transparent motion. J. Neurosci. 11, 2768–2785 (1991).
van Doorn, A. J. & Koenderink, J. J. Spatial properties of the visual detectability of moving spatial white noise. Exp. Brain Res. 45, 189–195 (1982).
Banton, T. & Levi, D. M. The perceived strength of motion-defined edges. Perception 22, 1195–1204 (1993).
Sachtler, W. L. & Zaidi, Q. Visual processing of motion boundaries Vision Res. 35, 807–826 (1995).
Maunsell, J. H. R. & van Essen, D. C. The connections of the middle temporal visual area (MT) and their relationship to a cortical hierarchy in the macaque monkey. J. Neurosci. 3, 2436–2586 (1983).
Born, R. T. & Tootell, R. B. H. Segregation of global and local motion processing in primate middle temporal visual area. Nature 357, 497–499 (1992).
Schiller, P. H. The effects of V4 and middle temporal (MT) area lesions on visual performance in the rhesus monkey. Vis. Neurosci. 10, 717–746 (1993).
Marcar, V. L., Xiao, D. K., Raiguel, S. E., Maes, H. & Orban, G. A. Processing of kinetically defined boundaries in the cortical motion area MT of the macaque monkey. J. Neurophysiol. 74, 1258–1270 (1995).
Britten, K. H., Shadlen, M. N., Newsome, W. T. & Movshon, J. A. Responses of neurons in macaque MT to stochastic motion signals. Vis. Neurosci. 10, 1157–1169 (1993).
Kwong, K. K. et al. Dynamic magnetic resonance imaging of human brain activity during primary sensory stimulation. Proc. Natl Acad. Sci. USA 89, 5675–5679 (1992).
Ogawa, S. et al. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping with magnetic resonance imaging. Proc. Natl Acad. Sci. USA 89, 5951–5955 (1992).
DeYoe, E. A., Bandettini, P., Neitz, J., Miller, D. & Winans, P. Functional magnetic resonance imaging (fMRI) of the human brain. J. Neurosci. Meth. 54, 171–187 (1994).
Dale, A. M. & Sereno, M. I. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. J. Cogn. Neurosci. 5, 162–176 (1993).
Acknowledgements
We thank E. Adelson, S. Brown, M. Livingstone and W. van Duffel for comments on earlier versions of this manuscript. S. Macknik kindly loaned us the eye-tracking device used in some experiments. We are grateful for the technical support of T. Campbell, T. Davis, M. Foley and M. Vevea, and to all of our subjects. J.B.R. was supported by an HHMI predoctoral fellowship, and R.B.H.T. by the Human Frontiers program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Reppas, J., Niyogi, S., Dale, A. et al. Representation of motion boundaries in retinotopic human visual cortical areas. Nature 388, 175–179 (1997). https://doi.org/10.1038/40633
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/40633
This article is cited by
-
What do we see in pictures? The sensory individuals of picture perception
Philosophical Studies (2022)
-
Image Segmentation Based on Relative Motion and Relative Disparity Cues in Topographically Organized Areas of Human Visual Cortex
Scientific Reports (2019)
-
Second-order visual sensitivity in the aging population
Aging Clinical and Experimental Research (2019)
-
Revisiting the functional significance of binocular cues for perceiving motion-in-depth
Nature Communications (2018)
-
Cytoarchitectonic mapping of the human dorsal extrastriate cortex
Brain Structure and Function (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.