Abstract
Cholecystokinin (CCK) is the most abundant neuropeptide in the mammalian brain, and in man significant quantities are expressed in all regions of the brain.1, 2 Therefore, CCK has been implicated in a variety of CNS functions—such as feeding behavior, anxiety, analgesia and memory functions as well as psychiatric disease like panic disorder and schizophrenia (for review, see2, 3). Recently, a number of studies have indicated that a C−36 to T transition in the CCK gene promoter Sp1 element4 (Figure 1) is associated with alcoholism and withdrawal symptoms as well as panic disorder.5, 6, 7 Moreover, it has been proposed that the polymorphism plays a direct role in the pathogenesis of the disorders by decreasing the expression and synthesis of CCK peptides. The significance of these findings is still unclear and other studies have failed to demonstrate linkage between the polymorphism and alcoholism.8 In this study we examined the function of the C−36 to T transition in transcription of the human CCK gene. We demonstrate that substitution of the C−36 residue causes a slight reduction of Sp1 and Sp3 binding, but this has no effect on transcription in vivo. Moreover, no difference in the response to physiological stimuli was observed. Taken together the results show that the C to T polymorphism does not play a direct role in the pathogenesis of either alcoholism or panic disorder and that a putative association to these disorders is likely to be the result of co-segregation with a linked mutation.

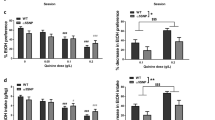
Structure of the proximal region of the human CCK promoter.4 The E-box, CRE/TRE, Sp1 and TATA box elements are indicated. The asterisk indicates the position of the C−36 to T transition and the arrow designates the site of transcriptional initiation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Rehfeld JF . Immunochemical studies on cholecystokinin. II. Distribution and molecular heterogeneity in the central nervous system and small intestine of man and hog J Biol Chem 1978; 253: 4022–4030
Rehfeld JF, Nielsen FC . Molecular forms and regional distribution of cholecystokinin in the central nervous system. In: Bradwein J, Vasar E (eds) Cholecystokinin and Anxiety Springer-Verlag: Heidelberg 1995; 33–56
Rehfeld JF . Cholecystokinin. In: Schultz SG, Makhlouf EM, Rauner D (eds) The Gastrointestinal System, Handbook of Physiology American Physiological Society: Bethesda, MD 1989; 337–358
Nielsen FC, Pedersen K, Hansen TVO, Rourke IJ, Rehfeld JF . Transcriptional regulation of the human cholecystokinin gene: composite action of upstream stimulatory factor, Sp1, and members of the CREB/ATF- AP-1 family of transcription factors DNA Cell Biol 1996; 15: 53–63
Wang Z, Valdes J, Noyes R, Zoega T, Crowe RR . Possible association of a cholecystokinin promotor polymorphism (CCK-36CT) with panic disorder Am J Med Genet 1998; 81: 228–234
Harada S, Okubo T, Tsutsumi M, Takase S, Muramatsu T . A new genetic variant in the Sp 1 binding cis-element of cholecystokinin gene promoter region and relationship to alcoholism Alcohol Clin Exp Res 1998; 22: 93S–96S
Okubo T, Harada S, Higuchi S, Matsushita S . Genetic association between alcohol withdrawal symptoms and polymorphism of CCK gene promoter Alcohol Clin Exp Res 1999; 23: 11S–12S
Ishiguro H, Saito T, Shibuya H, Toru M, Arinami T . No association between C-45T polymorphism in the Sp1 binding site of the promoter region of the cholecystokinin gene and alcoholism Psychiatry Res 1999; 85: 209–213
Hansen TVO, Bundgaard J, Nielsen F, Rehfeld J . Composite action of three GC/GT boxes in the proximal promoter region is important for gastrin gene transcription Mol Cell Endocrinol 1999; 155: 1–8
Hansen TVO, Rehfeld JF, Nielsen FC . Mitogen-activated protein kinase and protein kinase: a signaling pathway stimulates cholecystokinin transcription via activation of cyclic adenosine 3′,5′-monophosphate response element-binding protein Mol Endocrinol 1999; 13: 466–475
Letovsky J, Dynan WS . Measurement of the binding of transcription factor Sp1 to a single GC box recognition sequence Nucleic Acids Res 1989; 17: 2639–2653
Burley SK, Roeder RG . Biochemistry and structural biology of transcription factor IID (TFIID) Annu Rev Biochem 1996; 65: 769–799
Lydiard RB, Ballenger JC, Laraia MT, Fossey MD, Beinfeld MC . CSF cholecystokinin concentrations in patients with panic disorder and in normal comparison subjects Am J Psychiatry 1992; 149: 691–693
Bowen T, Norton N, Jacobsen NJ, Guy C, Daniels JK, Sanders RD et al. Linked polymorphisms upstream of exons 1 and 2 of the human cholecystokinin gene are not associated with schizophrenia or bipolar disorder Mol Psychiatry 1998; 3: 67–71
Rehfeld JF . How to measure cholecystokinin in tissue, plasma and cerebrospinal fluid Regul Pept 1998; 78: 31–39
Courey AJ, Tjian R . Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif Cell 1988; 55: 887–898
Hagen G, Muller S, Beato M, Suske G . Cloning by recognition site screening of two novel GT box binding proteins: a family of Sp1 related genes Nucleic Acids Res 1992; 20: 5519–5525
Andrews NC, Faller DV . A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells Nucleic Acids Res 1991; 19: 2499
Bradford MM . A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding Anal Biochem 1976; 72: 248–254
Schneider I . Cell lines derived from late embryonic stages of Drosophila melanogaster J Embryol Exp Morphol 1972; 27: 353–365
Di Nocera PP, Dawid IB . Transient expression of genes introduced into cultured cells of Drosophila Proc Natl Acad Sci USA 1983; 80: 7095–7098
Chen C, Okayama H . High-efficiency transformation of mammalian cells by plasmid DNA Mol Cell Biol 1987; 7: 2745–2752
Gorman CM, Moffat LF, Howard BH . Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells Mol Cell Biol 1982; 2: 1044–1051
Acknowledgements
Guntram Suske and Robert Tjian are gratefully acknowledged for the gift of plasmids. We thank Ria Petersen for skilfull technical assistance. Jan Christiansen is thanked for critically reading the manuscript. This study was supported by grants from the Danish Medical Research Council, the Danish Biotechnology Program for Signal Peptide Research, the John and Birthe Meyer Foundation and the NOVO Nordisk Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hansen, T., Rehfeld, J. & Nielsen, F. Function of the C−36 to T polymorphism in the human cholecystokinin gene promoter. Mol Psychiatry 5, 443–447 (2000). https://doi.org/10.1038/sj.mp.4000705
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4000705
Keywords
This article is cited by
-
Polymorphisms in the 5′-untranslated region of the human serotonin receptor 1B (HTR1B) gene affect gene expression
Molecular Psychiatry (2003)
-
Neuropeptide gene polymorphisms and human behavioural disorders
Nature Reviews Drug Discovery (2003)
-
Identification of a compound short tandem repeat stretch in the 5′-upstream region of the cholecystokinin gene, and its association with panic disorder but not with schizophrenia
Molecular Psychiatry (2001)