Abstract
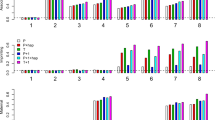
Family-based linkage disequilibrium mapping using SNP markers is expected to be a major route to the identification of susceptibility alleles for complex diseases. However there are a number of methodological issues yet to be resolved, including the handling of extended haplotype data and analysis of haplotype transmission in sib-pair or family trio samples. In the present study, we have analysed two dinucleotide repeat and six SNP markers at the COMT locus at chromosome 22q11, a region implicated in psychosis, for transmission distortion in 198 Chinese schizophrenic family trios. When individual markers were analysed using the TDT, two showed modest evidence of transmission distortion (186C/T, P = 0.04; Val158Met, P = 0.01). Using haplotypes of paired markers analysed by the program TRANSMIT, the most significant P value was 0.001, for the Met158Val and 900ins/delC polymorphisms in the COMT gene. The global P value for the haplotypes of all six SNP markers tested was 0.004, largely a result of the excess transmission of two extended haplotypes which differed at the marker 408C/G. The exclusion of this marker from the analysis gave a global P value of 0.002 and produced a five marker haplotype system which was significant at P = 0.0006. This haplotype consisted of the alleles −287G:186C:Val158:900insC:ARVCF930C, which may represent a background haplotype for the transmission of a schizophrenia susceptibility allele at chromosome 22q11. Our results support the hypotheses that either COMT is itself a susceptibility gene, or more likely that this region of chromosome 22 contains a susceptibility gene that is in linkage disequilibrium with COMT alleles.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Lander ES, Schork NJ . Genetic dissection of complex traits Science 1994; 265: 2037–2048 Erratum 266: 353
Lander E, Kruglyak L . Genetic dissection of complex traits: guidelines for interpreting and reporting linkage results Nat Genet 1997; 11: 241–247
Risch N, Merikangas K . The future of genetic studies of complex human diseases Science 1996; 273: 1516–1517
Lander ES . The new genomics: global views of biology Science 1996; 274: 1594–1595
Collins FS, Guyer MS, Chakravati A . Variations on a theme: cataloguing human genetic variation Science 1997; 278: 1580–1581
Sham PC . Statistics in Human Genetics Arnold, UK 1998
Shork NJ, Cardon L, Xu X . The future of genetic epidemiology TIGS 1998; 14: 266–271
Copeman JB, Cucca F, Hearne CM, Cornall RJ, Reed PW, Ronningen KS et al. Linkage disequilibrium mapping of a type 1 diabetes susceptibility gene (IDDM7) to chromosome 2q31–q33 Nat Genet 1995; 9: 80–85
Pritchard LE, Kawaguchi Y, Reed PW, Copeman JB, Davies JL, Barnett AH et al. Analysis of the CD3 gene region and type I diabetes: application of fluorescence-based technology to linkage disequilibrium mapping Hum Mol Genet 1995; 4: 197–202
Nickerson DA, Taylor SL, Weiss KM, Clark AG, Hutchinson RG, Stengard J et al. DNA sequence diversity in a 9.7-kb region of the human lipoprotein lipase gene Nat Genet 1998; 19: 233–240
Chakravati A . It's raining SNPs, hallelujah Nature Genet 1998; 19: 216–217
Scambler P, Kelly D, Lindsay E, Williamson R, Goldberg R, Shprintzen R et al. Velo-cardio-facial syndrome associated with chromosome 22 deletions which encompass the DiGeorge syndrome locus Lancet 1992; 339: 1138–1139
Dunham I, Collins I, Wadey R, Scambler P . Possible role for COMT in psychosis associated with velo-cardio-facial syndrome Lancet 1992; 340: 1361–1362
Shprintzen RJ, Goldberg R, Golding-Kushner KJ, Marion R . Late-onset psychosis in the velo-cardio-facial syndrome Am J Med Genet 1992; 42: 141–142
Pulver AE, Nestadt G, Goldberg R, Shprintzen RJ, Lamacz M, Wolyniec PS et al. Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives J Nerv Ment Dis 1994; 182: 476–478
Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, Borrow J et al. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11 Proc Natl Acad Sci USA 1995; 92: 7612–7616
Carlson C, Papolos D, Pandita RK, Faedda GL, Veit S, Goldberg R et al. Molecular analysis of velo-cardio facial syndrome patients with psychiatric disorders Am J Hum Genet 1997; 60: 851–859
Yan W-L, Jacobsen LK, Krasnewich DM, Guan X-Y, Lenane MC, Paul SP et al. Chromosome 22q11.2 interstitial deletions among childhood-onset schizophrenics and multi-dimensionally impaired Am J Med Genet Neuropsychiatr Genet 1998; 81: 41–43
Karayiorgou M, Gogos JA . Dissecting the genetic complexity of schizophrenia Mol Psychiatry 1997; 2: 211–223
Lachman D, Kelsoe JR, Remick R, Sadovnick AD, Rapaport MH, Lin M et al. Linkage studies suggest a possible locus for bipolar affective disorder near the VCF syndrome region on chromosome 22 Am J Med Genet Neuropsychiatr Genet 1997; 74: 121–128
Edenberg HJ, Foroud T, Conneally PM, Sorbel JJ, Carr K, Crose C et al. Initial genomic scan of the NIMH genetics initiative bipolar pedigrees: chromosomes 3, 5, 15, 16, 17, and 22 Am J Med Genet Neuropsychiatr Genet 1997; 74: 238–246
Kelsoe JR, Loetscher E, Spence MA, Foguet M, Sadovnick AD, Remick RA et al. A genome survey of bipolar disorder indicates a susceptibility locus on chromosome 22 Am J Med Genet 1998; 8: 461–462
Shaw SH, Kelly M, Smith AB, Shields G, Hopkins PJ, Loftus J et al. A genome wide search for schizophrenia susceptibility genes Am J Med Genet Neuropsychiatr Genet 1998; 81: 364–376
Baron M, Gruen R, Levitt M, Hunter C, Asnis L . Erythrocyte catechol O-methyltransferase activity in schizophrenia: analysis of family data Am J Psychiatry 1984; 141: 29–32
Lotta T, Vidgren J, Tilgmann C, Ulmanen I, Melen K, Julkunen I et al. Kinetics of human soluble and membrane-bound catechol O-methyltransferase: a revised mechanism and description of the thermolabile variant of the enzyme Biochemistry 1995; 34: 4202–4210
Lachman D, Papolos DF, Saito T, Yu YM, Szumlanski CL, Weinshilboum RM . Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders Pharmacogenetics 1996; 6: 243–250
Li T, Sham PC, Vallada HP, Xie T, Tang X, Liu X et al. Preferential transmission of the high activity allele of COMT in schizophrenia Psychiatric Genet 1996; 6: 131–133
McLeod HL, Fang L, Luo X, Scott EP, Evans WE . Ethnic differences in erythrocyte catechol-O-methyltransferase activity in black and white Americans J Pharmacol Exp Ther 1994; 270: 26–29
Guiterrez B, Bertranpetit J, Guillamat R, Valles V, Arranz MJ, Kerwin RW et al. Association analysis of the catechol-O-methyltransferase gene and bipolar affective disorder Am J Psychiatry 1996; 154: 113–115
Daniels JK, Williams NM, Williams J, Jones LA, Cardno AG, Murphy KC et al. No evidence for allelic association between schizophrenia and a polymorphism determining high or low catechol-O-methyltransferase activity Am J Psychiatry 1996; 153: 268–270
Karayiorgou M, Gogos JA, Galke BL, Wolyniec PS, Nestadt G, Antonarakis SE et al. Identification of sequence variants and analysis of the role of the catechol-O-methyltransferase gene in schizophrenia susceptibility Biol Psychiatry 1998; 43: 425–431
BIOMED European Bipolar Collaborative Group . No association between bipolar disorder and alleles at a functional polymorphism in the COMT gene Br J Psychiatry 1997; 170: 526–528
Kirov G, Murphy KC, Arranz MJ, Jones I, McCandles F, Kunugi H et al. Association of a functional polymorphism of the catechol-O-methyltransferase gene with rapid cycling bipolar disorder Mol Psychiatry 1997; 3: 342–345
Gogos JA, Morgan M, Luine V, Santha M, Ogawa S, Pfaff D et al. Catechol-O-methyltransferase-deficient mice exhibit sexually dimorphic changes in catecholamine levels and behaviour Proc Natl Acad Sci USA 1998; 95: 9991–9996
Lachman HM, Nolan KA, Mohr P, Saito T, Volavka J . Association between catechol O-methyltransferase genotype and violence in schizophrenia and schizoaffective disorder Am J Psychiatry 1998; 155: 835–837
Strous RD, Bark N, Parsia SS, Volavka J, Lachman HM . Analysis of a functional catechol-O-methyltransferase gene polymorphism in schizophrenia: evidence for association with aggressive and antisocial behavior Psychiatry Res 1997; 24: 71–77
Papalos DF, Veit S, Faedda GL, Saito T, Lachman HM . Ultra-ultra rapid cycling bipolar disorder is associated with the low activity allele of catechol-O-methyltransferase Mol Psychiatry 1998; 3: 346–349
Kunugi H, Vallada HP, Sham P, Hoda F, Arranz MJ, Nanko S et al. Catechol-O-methyltransferase polymorphisms and schizophrenia: a transmission disequilibrium study in multiply affected families Psychiatric Genet 1997; 7: 97–102
Tenhunen J, Salminen M, Lundstrom K, Kiviluoto T, Savolainen R, Ulmanen I . Genomic organisation of the human catechol O-methyltransferase gene and its expression from two distinct promoters Eur J Biochem 1994; 223: 1049–1059
Orita M, Iwahana H, Kanazawa H, Hayashi K, Sekiya T . Detection of polymorphisms of human DNA by gel electrophoresis as single-strand conformation polymorphisms Proc Natl Acad Sci USA 1989; 86: 2766–2770
Glavac D, Dean M . Optimization of the single-strand conformation polymorphism (SSCP) technique for detection of point mutations Hum Mutat 1993; 2: 404–414
Sirotkin H, O'Donnell H, DasGupta R, Halford S, St Jore B, Puech A et al. Identification of a new human catenin gene family member (ARVCF) from the region deleted in velo-cardio-facial syndrome Genomics 1997; 41: 75–83
Hoda F, Chaudhuri KR, Arranz M, Aitchison KJ, Al-Chalabi A, Kunugi H et al. Parkinson's disease and low activity alleles of COMT Biochem Biophys Res Comm 1995; 228: 780–784
Long JC, Williams RC, Urbanek M . An E–M algorithm and testing strategy for multiple-locus haplotypes Am J Hum Genet 1996; 56: 799–810
Klitz W, Stephens JC, Grote M, Carrington M . Discordant patterns of linkage disequilibrium in the peptide transporter loci within the HLA class II region Am J Hum Genet 1995; 57: 1436–1444
Spielman RS, McGinnis RE, Ewens WJ . Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM) Am J Hum Genet 1993; 52: 506–516
Sham PC, Curtis D . An extended transmission/disequilibrium test (TDT) for multi-allele marker loci Ann Hum Genet 1995; 59: 323–336
Clayton DG . TRANSMIT v2.3 1998http://watson.hgen.pitt.edu/docs/transmit.html
Chiano MN, Clayton DG . Fine genetic mapping using haplotype analysis and the missing data problem Ann Hum Genet 1998; 62: 55–60
Hacia JG, Brody LC, Collins FS . Applications of DNA chips for genomic analysis Mol Psychiatry 1998; 3: 483–492
Jorde LB, Watkins WS, Viskochil D, O'Connell P, Ward KJ . Linkage disequilibrium in the neurofibromatosis I (NF1) region: implications for gene mapping Am J Hum Genet 1993; 53: 1038–1050
Terwilliger JD, Ott J . Linkage disequilibrium between alleles at marker loci In: Handbook of Human Genetic Linkage, 1st edn Johns Hopkins University Press: Baltimore 1994; pp 188–198
Acknowledgements
We are grateful for a NARSAD independent investigator Award to DAC, which principally funded this work, and for a Wellcome Trust Biomedical Research Collaboration grant to DAC and XL. We are also grateful for the support of the Wellcome Trust to PCS and JZ, and the China National Natural Sciences Foundation, The Doctoral Fund of the Chinese National Committee of Education and the New York China Medical Board (TL).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, T., Ball, D., Zhao, J. et al. Family-based linkage disequilibrium mapping using SNP marker haplotypes: application to a potential locus for schizophrenia at chromosome 22q11. Mol Psychiatry 5, 77–84 (2000). https://doi.org/10.1038/sj.mp.4000638
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.mp.4000638