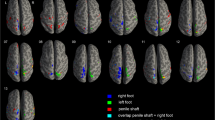
Abstract
It is well known that penile erection is dependent on commands from the central nervous system. However, there has been little research on the central control of penile erection. The aim of this study was to evaluate, for the first time, the cerebral centers of penile erection using BOLD-functional MRI. Functional magnetic resonance imaging (fMRI) on a 1.5T MR scanner was performed in 12 sexually potent male volunteers (mean age: 23) and two hypogonadal impotent patients. In this study, blood oxygenation level dependent (BOLD) technique was utilized to create fMRI reflecting local brain activities. Real-time visual stimulation was performed with an alternatively combined erotic and non-erotic film to identify and quantify the activated brain regions associated with sexual response. Subjective sexual arousal and penile erection responses were assessed using 5-point scales ranging from 1 (no change) to 5 (maximal increase). In normal volunteers, the mean scores on subjective sexual arousal and penile erection by sexual stimulation with erotic film were 3.0 and 3.3 respectively, whereas there were no changes by non-erotic stimulation. During the visual stimulation the occipital cortex was activated by either an erotic or non-erotic film, the erotic film gave 150–200% stronger activation. However, more than seven of the 12 healthy subjects were significantly activated in the areas of inferior frontal lobe, cingulate gyrus, insula gyrus, corpus callosum, thalamus, caudate nucleus, globus pallidus, and inferior temporal lobe by erotic stimulation. In the hypogonadal patients, brain activation in response to the erotic film decreased compared to normal volunteers, however, it was restored by testosterone supplementation. These results are the first demonstration to show the functional neuroanatomy of the brain associated with sexual arousal by visual sexual stimulation using BOLD-based fMRI. Further studies are needed to verify that fMRI provides an important new tool in evaluating the cerebral center of the penile erection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 8 print issues and online access
$259.00 per year
only $32.38 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Lue TF. . Drug therapy; erectile dysfunction. New Engl J Med 2000 342, 1802–1813.
Chuang AT, Steers WD. . Neurophysiology of the penile erection. In: Carson C, Kirby R, Goldstein L (eds). Textbook of Erectile Dysfunction. ISIS Medical Book: Oxford 1999 59–72.
Marson L. . Central nervous system control. In: Carson C, Kirby R, Goldstein L (eds). Textbook of Erectile dysfunction. ISIS Medical Book: Oxford 1999 73–88.
McKenna KE. . Central control of penile erection. Int J Impot Res 1998 10(Suppl), S25–S34.
McKenna K. . The brain is the master organ in sexual function: central nervous system control of male and female sexual function. Int J Impot Res 1999 11(Suppl), S48–S55.
Giuliano F et al.Stimulation of the medial preoptic area of the hypothalamus in the rat elicits increases in intracavernous pressure. Neurosci Lett 1996 209, 1–4.
Shimura T, Yamamoto T, Shimokochi M. . The medial preoptic area is involved in both sexual arousal and performance in male rats; re-evaluation of neuron activity in freely moving animals. Brain Res 1994 640, 215–222.
George MS, Ketter TA, Post RM. . SPECT and PET imaging in mood disorders. J Clin Psychiatry 1993 54, 6–13.
Stoleru S et al.Neuroanatomical correlates of visually evoked sexual arousal in human males. Archives of Sexual Behavior 1999 28, 1–21.
Ring HA, George M, Costa DC, Ell PJ. . The use of cerebral activation procedures with single photon emission tomography. Eur J Nucl Med 1991 18, 133–141.
Stehling MK, Turner R, Mansfield P. . Echo-planar imaging; magnetic resonance imaging in a fraction of a second. Science 1991 254, 43–50.
Longworth C, Honey G, Sharma T. . Functional magnetic resonance imaging in neuropsychiatry. Br J Med 1999 319, 1551–1554.
Ogawa S et al.Barin magnetic resonance imaging with contrast dependent on blood oxygenation. Proc Natl Acad Sci USA 1990 87, 9868–9872.
Cho ZH et al.New findings of the correlation between acupoints and corresponding brain cortices using functional MRI. Proc Natl Acad Sci USA 1998 95, 2670–2673.
Nemeroff CB, Kilts CD, Berns GS. . Functional brain imaging; twenty-first century phrenology or psychobiological advance for the millennium. Am J Psychiatry 1999 156, 671–673.
Villringer A, Dirnagl U. . Coupling of brain activity and cerebral blood flow; basis of functional neuroimaging. Cerebrovasc Brain Metab Rev 1995 7, 240–276.
Morgan JI, Cohen DR, Hempstead, JL, Curran T. . Mapping patterns of c-fos expression in the central nervous system after seizure. Science 1987 237, 192–196.
Kuypers HCJM, Ugolina G. . Viruses as transneuronal tracers. Trends Neurosci 1990 13, 71–75.
Coolen LM, Peters HJPW, Veening JG. . Fos immunoreactivity in the rat brain following cosummatory elements of sexual behavior, a sex comparison. Brain Res 1996 738, 67–82.
Marson L, Platt KB, McKenna KE. . Central nervous system innervation of the penis as revealed by the transneuronal transport of pseudorabies virus. Neuroscience 1993 55, 263–280.
Chen KK, Chan SHH, Chang LS, Chan JYH. . Participation of paraventricular nucleus of hypothalamus in central regulation of penile erection in the rat. J Urol 1997 158, 238–244.
Guyton AC. . Basic Neuroscience; Anatomy and Physiology. Saunders: Philadelphia 1991 254–264.
Meisel RL, Sachs BD. . The physiology of male sexual behavior. In: Knobil E, Neill JD (eds). The Physiology of Reproduction. Raven Press: New York 1994 3–104.
Simerly RB, Swanson LW. . The organization of neural inputs to the medial preoptic nucleus of the rat. J Comp Neurol 1986 246, 312–342.
Sar M, Stumpf WE. . Distribution of androgen concentrating neurons in the rat brain. In: Stumpf WE, Grant LD (eds). Anatomical Neuroendocrinology. Karger: Basel 1975 120–133.
Maillard-Gutekunst CA, Edwards DA. . Preoptic and sub-thalamic connections with the caudal brainstem are important for copulation in the male rat. Behavioral Neuroscience 1994 108, 758–766.
Devinsky O, Morrell MJ, Vogt BA. . Contributions of anterior cingulate cortex to behaviour. Brain 1995 118, 279–306.
Goldenberg G et al.Contributions of occipital and temporal brain regions to visual and acoustic imagery-a spect study. Neuropsychologia 1991 29, 695–702.
Cunningham GR, Hirshkowitz M. . Androgen deficiency. In: Hellstrom WJG (ed). Male Infertility and Sexual Dysfunction. Springer: New York 1997 340–355.
Greco B, Edwards DA, Michael RP, Clancy AN. . Androgen receptor immunoreactivity and mating-induced Fos expression in forebrain and midbrain structures in the male rat. Neuroscience 1996 75, 161–171.
Tiihonen J et al.Increase in cerebral blood flow of right prefrontal cortex in man during orgasm. Neuroscie Lett 1994 170, 241–243.
Nour S et al.Cerebral activation during micturation in normal men. Brain 2000 123, 781–789.
Acknowledgements
This work was supported by a grant from Chonnam National University in the year of 2000.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Park, K., Seo, J., Kang, H. et al. A new potential of blood oxygenation level dependent (BOLD) functional MRI for evaluating cerebral centers of penile erection. Int J Impot Res 13, 73–81 (2001). https://doi.org/10.1038/sj.ijir.3900649
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.ijir.3900649
Keywords
This article is cited by
-
Sexual function in men undergoing androgen deprivation therapy
International Journal of Impotence Research (2021)
-
The role of anterior and posterior insula in male genital response and in visual attention: an exploratory multimodal fMRI study
Scientific Reports (2020)
-
Neural substrates of sexual arousal in heterosexual males: event-related fMRI investigation
Journal of Physiological Anthropology (2016)
-
Sex differences in interactions between nucleus accumbens and visual cortex by explicit visual erotic stimuli: an fMRI study
International Journal of Impotence Research (2015)
-
Erectile dysfunction (ED) after ischemic stroke: association between prevalence and site of lesion
Clinical Autonomic Research (2015)