Abstract
The pathogenic influence of viral agents in chronic inflammatory joint diseases like rheumatoid arthritis has been discussed for many years. More recently, DNA of several viruses, among them parvovirus B19 (B19), was traceable by PCR analysis in synovial fluid and synovial tissue. To investigate the potential role of parvovirus B19 in rheumatoid arthritis, we analyzed the expression of B19 VP1/VP2 proteins by immunohistochemistry in paraffin sections of 63 synovial specimens in rheumatoid arthritis (RA; n = 29), psoriatic arthritis (PSA; n = 6), nonspecific arthritis or synovitis (n = 26), and normal synovia (n = 2). Thereby we could demonstrate replicative virus infection in a variable number of cells in about 90% of rheumatoid specimens and in four of six (66%) cases of psoriatic arthritis, but only in 38% of cases with chronic reactive inflammation and one case of normal synovia. In virus-positive rheumatoid specimens, moreover, the average number of affected cells was significantly higher than in virus-expressing synovia of nonspecific reactive inflammation. These findings support the importance of B19-viral infection in the pathogenesis of chronic arthritis. B19-positive cells in the synovia could be ascribed to CD20- or CD3-positive B- or T-lymphocytes by double immunostaining. Based on these results, B19 infection of lymphocytic cells also seems possible.
Similar content being viewed by others
INTRODUCTION
Virus infections are a well-known major cause of arthritis in humans (1). Appearing preferentially as a sequela to the systemic viral disease, arthritic symptoms in many instances are supposed to be due to autoimmunologic cross-reaction in immune response toward viral epitopes. In some conditions, however, lytic infection of the joint has been described for different viruses (2, 3).
A pathogenic influence of viral agents, especially EBV and parvovirus B19, has likewise long been discussed in chronic inflammatory joint diseases like rheumatoid arthritis (3, 4, 5, 6, 7).
DNA of parvovirus B19 and several other viruses, for instance, EBV and CMV, could be detected in a high percentage of cases in rheumatoid synovial tissue and synovial fluid by highly sensitive PCR analysis (8, 9). A primary role in disease development, however, has still been questioned (3, 10, 11).
Parvovirus B19 is the causative agent in erythema infectiosum (12). Among adults there is a high seroprevalence of 50–70% in the population (13, 14). Specifically affecting erythroid progenitor cells by means of erythroid P antigen (15, 16) in healthy adults, it can lead to mainly mild or subclinical symptoms of anemia, thrombopenia, or granulocytopenia, but also aplastic crisis or complicating hemolytic anemias (17, 18, 19). Apart from hematology it has come to special notice in prenatal medicine, because prenatal parvovirus B19-infection in the second trimester of pregnancy, when major fetal hematopoiesis takes place, can cause dramatic anemia and fatal hydrops of the fetus (20, 21, 22). Furthermore a frequent association of B19 with acute, usually self-limiting arthropathy has been reported (23, 24). Recent data, hinting at the persistence of parvovirus B19 in the joint (7, 25, 26) now focus further attention on this virus. Questions to be addressed in this context concern the possible involvement of parvovirus B19 in disease origin, maintenance, or progression in immune-mediated or reactive chronic arthritis.
Earlier data on B19 persistence in synovial tissue were mainly based on DNA analyses. To elucidate the role of parvovirus B19 in chronic inflammatory joint disease, we investigated the expression of immunogenic B19 capsid proteins (VP1/VP2) by immunohistochemical analysis in paraffin sections of 63 synovial specimens of rheumatoid arthritis (RA), psoriatic arthritis (PSA), reactive synovitis/arthritis including osteoarthritis, and normal synovia.
MATERIALS AND METHODS
Sixty-three synovial specimens were obtained from the Department of Pathology and the Department of Orthopedic Surgery of Saarland University, Homburg, the Department of Pathology of the Central Hospital in Augsburg, the Department of Orthopedic Surgery of the Bundesknappschaft’s Hospital Puettlingen, and the Department of Rheumaorthopedics of the Rheumazentrum Oberammergau. Synovial tissue had been removed either surgically or by arthroscopy for therapeutic or diagnostic reasons. Normal synovia was gained by diagnostic arthroscopy in traumatic joint lesion or by autopsy (Department of Forensic Medicine of Saarland University, Homburg). In prospectively-collected cases, informed consent for research use of the tissues was obtained; in retrospectively-analyzed tissue specimens of anonymized archival material, general consent for scientific use of diagnostic material was ensured. Cases included in this study are 29 rheumatoid arthritis (RA; 20 f/9 m), 26 chronic reactive synovitis/arthritis (14 f/12 m), 6 psoriatic arthritis (PSA; 6 m), and 2 normal synovial specimens (1 f/1 m). Among chronic reactive synovitis/arthritis cases, we compiled synovial specimens of osteoarthritis from arthritic joints, detritus arthritis, chronic irritation after joint injuries, and unclear chronic synovitis, without clinical diagnosis of rheumatoid arthritis. The criteria for the clinical diagnosis of rheumatoid arthritis and psoriatic arthritis were based on the revised American College of Rheumatology (ACR; formerly, the American Rheumatism Association) (27); the criteria for diagnoses of reactive arthritides were the exclusion of any specific or systemic arthritis, the lack of diagnostic criteria of rheumatoid or psoriatic arthritis, or the clear presence of posttraumatic changes. Synovial tissue, in descending frequencies, was derived mainly from knee, hip, carpal, or tarsal joints. Primary synovial cell cultures were established in a subset of cases, where vital tissue was obtained. Because the majority of cases were derived from archival material, from which it was impossible to obtain serologic data, the serologic analysis on parvovirus B19 (IgM and IgG) had to be dispensed with in this study.
Immunohistochemistry
For parvovirus B19 detection a monoclonal mouse antibody reactive to parvovirus B19 VP1 and VP2 capsid protein was used, kindly provided by Dade Behring (Marburg, Germany). VP1 (structural protein 1) and VP2 (structural protein 2) genes overlap, whereas VP2 open reading frame is totally included within the VP1 open reading frame (28, 29). The antibody used recognizes VP1 as well as VP2 protein. Kidney tissue of a B19-infected hydropic fetus, analyzed earlier by B19-DNA in situhybridization was used as positive control; by antibody staining, B19-positive cells were specifically detected to the same extent and with the same histological distribution as by in situ hybridization, either intravasal or in areas of fetal hematopoiesis (22). Immunohistochemical investigation of B19-negative fetal kidney control tissue did not reveal positive staining of cells. Immunohistochemical analysis of synovial as well as control tissues was performed on routinely processed formalin-fixed paraffin sections. After dewaxing in xylene and rehydration in a descending alcohol series, tissue sections were subjected to a proteolytic treatment in 0.1% pronase (Protease Type XXIV, Sigma) in PBS (6–8 min, RT) followed by application of parvovirus B19 VP1/VP2-antibody (1:100 in 1%BSA/PBS) and incubation for 1 hour at RT. Finally, the primary antibody was detected by alkaline phosphatase-mediated NBT staining using secondary antibody steps according to the APAAP protocol. In positive cases, control experiments omitting the primary VP1/VP2 antibody as well as absorbing the monoclonal antibody with VP1 and VP2 protein by preincubation revealed negative staining results.
Cell Type Characterization
Characterization of cell types was performed using a B-cell-specific mouse monoclonal CD20 antibody (DAKO; 1:30) and a T-cell specific mouse monoclonal CD3 antibody (Novocastra; 1:100). For immuno double staining after the above-mentioned VP1/VP2 detection, CD20 or CD3 labeling was performed by peroxidase-mediated AEC staining using the automated Nexis program (Ventana).
Cell Culture
Primary cell cultures were established in prospectively collected synovial specimens. After mechanical dissection, primary tissue material was subjected to collagenase treatment for 12–16 hours. Disaggregated cells were cultured in Dulbecco’s modified Eagle Medium (DMEM, GibcoBRL) supplemented with 10% FCS, 1 × penicillin/streptomycin (GibcoBRL), and 1 × nonessential amino acids (GibcoBRL) for several passages under standard cell-culture conditions. For immunofluorescence staining, cells of Passage 2–3 were grown in situ on sterile glass slides and fixed in methanol.
RESULTS
Parvovirus B19 capsid protein, indicating a replicative virus infection, was identified by immunohistochemical analysis in 63.4% (n = 40) of arthritic specimens (65%, n = 41, including normal synovial specimen). In detail, B19-positive cells were detected in 89.7% (n = 26) of rheuma cases, 66% (four of six cases) of psoriatic arthritis, 38.5% (n = 10) of nonspecific synovitis, and in one of the two normal synovial specimens (Table 1). Among individual virus-positive cases, the number of affected cells varied widely, leading to semiquantitative classification into three groups representing the different extent of virus multiplication (Table 2):
-
Group I (+): single virus-positive cells (Fig. 1D);
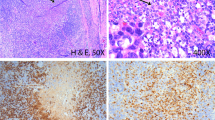
FIGURE 1 Parvovirus B19 immunohistochemistry in synovial tissue sections; detected virus protein is NBT labeled (purple black). A, rheumatoid arthritis specimen showing numerous positive cells (Group III; 400 ×). B, reactive chronic synovitis (Case 46) with many B19-positive cells assigned to Group III (400 ×). C, rheumatoid synovia with several positive cells (Group II); and D, rheumatoid arthritis showing only single virus-expressing cells (Group I; 400 ×).
-
Group II (++): several positive cells (Fig. 1C);
-
Group III (+++): numerous positive cells (Fig. 1, A–B).
B19-positive reactive synovitis and arthritis cases (n = 10) were mainly classified into Group I (4/10) or with inhomogeneous distribution between I and II (3/10); two cases were classified into Group II, and one case into Group III.
The four B19-positive PSA cases were assigned to Group I (2/4), Group II (1/4), and Group III (1/4). The majority of the B19-positive RA cases (n = 26) were classified into Group II (9/26 = 34.6%) and III (11/26 = 42.3%) in nearly equal percentages; four cases (15.4%) were assigned to Group I, two cases (7.7%) between Groups I and II (regionally III) because of inhomogeneous distribution of positive cells.
One normal synovial tissue (Case 63) was found to be B19 positive with several isolated positive cells defined as Group I/II.
Cases with a high number of virus-positive cells comprising Group III (Fig. 1, A–B) were almost exclusively found among rheumatic and, in one case, psoriatic synovial specimens. A similarly high number of parvovirus B19-infected cells could be identified in only one case of reactive arthritis of unknown origin (Case 46; Fig. 1B), whereas all other virus-positive cases of reactive chronic synovitis/arthritis were classified into Group I, I/II, or II. B19 VP1/VP2 protein was mainly detected in cells within the synovial stroma. Virus expression could not be observed in synovial lining cells or morphologically distinguishable synoviocytes. Also, few intravasal B19-positive cells were identified in synovial sections of individual specimens.
CD20 and CD3 immuno double-staining on paraffin sections of rheumatoid synovial tissue clearly identified parvovirus B19-positive cells as B and T lymphocytes; B19-positive cells were colocalized with areas of CD20- or CD3-positive cells (Fig. 2A) and showed double labeling of VP1/VP2 and CD20 (Fig. 2C) or CD3 (Fig. 2B).
Immuno double staining in synovial tissue sections. A, parvovirus B19 VP1/VP2 protein (NBT; purple black) and T-lymphocyte-specific CD3 (AEC; brown) in rheumatic synovial tissue (400 ×); B19-positive cells are colocalized with CD3-positive T lymphocytes. B, CD3 and B19 VP1/VP2 double-stained cells (630 ×). C, Parvovirus B19 VP1/VP2 protein (NBT; purple black) and B-lymphocyte-specific CD20 (AEC; brown) in rheumatic synovial tissue (630 ×); two B19–positive cells are characterized as B lymphocytes by CD20 double labeling.
In three cases of rheumatoid arthritis (Cases 1, 6, and 8) expressing parvovirus B19, primary synovial cell lines were established in addition to paraffin-embedded tissue. To test the prevalence and proportion of virus-positive cells in synovial cell cultures after several culture passages, immunofluorescence staining for parvovirus B19 protein was performed in Passage 2 or 3 on cells of respective cases cultivated in situ. B19-positive cells could not be detected in cultured synovial cells in any of the three cases.
DISCUSSION
Parvovirus B19-positive cells could be detected at an overall percentage of 63.5% in synovial tissue of chronic inflammatory joint disease. Surprisingly, the proportion of cases expressing virus protein was significantly higher in immune-related arthritis, especially rheumatoid arthritis (89.7%), and, to a lesser extent, psoriatic arthritis (66%), compared with reactive arthritis and synovitis (38.5%). Rheumatoid arthritis is considered as autoimmune arthritis, and the involvement of autoimmune processes is strongly supposed in psoriatic arthritis as well. The histomorphological characteristics of the two entities are quite distinctive but show clear similarities. The significant overrepresentation of virus-positive cases among the autoimmune arthritis specimens might well be caused by a defective immune response leading to a breakdown of virus repression in the chronically inflamed joint.
Because of culture conditions in primary synovial cell cultures, adherent synovial fibroblasts and dendritic cells were selected. Even in primary lymphocyte-rich tissue specimens, lymphocytes were mainly eliminated after several culture passages. No B19-protein-positive cells were identified in synovial cell cultures of virus-positive paraffin specimens, confirming their identity as T or B lymphocytes shown by immuno double staining. Lytic B19 infection via erythrocyte P antigen (glycolipoid globoside) (15) serving as its cellular receptor is known in cells of the erythroid lineage (15, 30) but—despite extensive lymphocyte response to B19 proteins—has not been described in lymphocytes. The infection of lymphocytes may be mediated by other cellular receptors (30). The finding of B19 capsid protein in lymphocytes in synovial tissue reported here indicates that virus replication is not restricted to the erythroid lineage.
In both rheumatoid arthritis and psoriatic arthritis, extensive infiltration of B and T lymphocytes (rheumatoid arthritis) or mainly Tlymphocytes (psoriatic arthritis) is observed (31). Because parvovirus B19-positive cells within the synovial tissue could be identified as lymphocytes, the difference in frequency of B19-positive cases, as well as the difference in the respective number of affected cells compared with reactive chronic arthritis specimens, could possibly be explained by varying total numbers of infiltrating lymphocytes.
Rheumatoid arthritis and psoriatic arthritis, in the natural course of disease, imply a chronic inflammation over a certain amount of time, which might give rise to much more profound disturbances, as observed in many cases of reactive chronic synovitis or arthritis. To exclude the possibility that the extent of chronic inflammation might be solely responsible for differences of parvovirus B19 expression between these two entities, especially cases with histomorphological signs of marked chronic changes and inflammation, were included among reactive arthritis/synovitis specimens. In individual analysis of this subgroup, no significant deviation from the summarized result of chronic reactive arthritis/synovitis cases was obvious, being inconsistent with the above-mentioned hypothesis of a purely quantitative effect.
VP1 and VP2 capsid proteins are both targets for serological immune response of IgG (VP1 and VP2) and IgM (mainly VP2) (32). Assuming an immune response to the expressed viral proteins in the joint, the observed findings unequivocally indicate a significant importance of parvovirus B19 in disease maintenance and progression in rheumatoid and psoriatic arthritis and to a lesser extent also in other forms of chronic inflammatory joint disease.
Moreover, the especially high percentage of >89% of virus-expressing cases among the rheumatoid specimens—compared with a seroprevalence of 50–70% in the population (13, 14)—probably hints at a causal role of parvovirus B19 even in the primary pathogenesis. Very interesting in this respect is the case of a 5-year-old (rheumatoid factor positive) RA patient analyzed in this study (Case 14); despite being one of the primary lesions, the synovia in these early stages of the general disease was already found to be heavily B19 positive.
Among cases with numerous virus-positive cells (Group III), there was only one case of reactive chronic arthritis (Case 46; Fig. 1B). The corresponding patient was a 20-year-old woman with a history of inconsistent, isolated unilateral knee problems over a period of about 1 year. Clinically the diagnosis had not been rheumatoid arthritis but unclear chronic synovitis or plica syndrome. Rheumatoid arthritis could not, however, be excluded by histopathological analysis in this case. The strong synovial parvovirus infection in this patient, who had clinically apparent symptoms for a comparatively short time, makes arthritis caused primarily by parvovirus B19 probable. In particular, the histomorphological findings similar to rheumatoid arthritis are suggestive of a parvovirus B19-triggered arthritis, which over the course of time might develop the clinical criteria for rheumatoid arthritis.
Among chronic reactive synovitis/arthritis specimens in three cases (Case 46, Case 36, Case 37), histomorphological findings similar or comparable to rheumatoid arthritis were described; all of them were B19 positive, showing the highest number of positive cells (1 Group III, 2 Group II) observed in the reactive arthritis cases. Thus, parvovirus B19 might especially be involved in the histomorphological rheuma phenotype.
Only two cases of normal synovial specimens were studied. Synovial tissue is hard to obtain in joints that are in truly normal condition. B19-expressing cells (Group I/II) were identified in one of the normal synovial specimens (Case 63). Anamnestically, a traumatic joint lesion in sports injury was reported. There was little clinical information, but no arthritic problems were mentioned. A chronic subclinical synovial irritation in this case, however, might nonetheless be possible. On the other hand, B19 protein expression could also be present in normal synovial tissue, according to reported DNA data (25, 26).
Parvovirus B19 is known to persist in bone marrow (33, 34) and probably in synovial tissue (25, 35). There is no indication of integration of viral genome or establishment of latency. Thus the persistence is due to ongoing low-level virus replication in bone marrow cells (32) and most likely cells within the synovial tissue. In synovial lining cells, mainly three different subtypes can be distinguished: Type A synoviocytes (bone marrow-derived cells with monocyte and mature macrophage characteristics), Type B synoviocytes (fibroblastoid cells), and dendritic cells. Whether and to what extent B19 also persists in synovial lining cells in the normal synovial tissue or whether there is recruitment of B19-positive lymphocytes only infiltrating from the bone marrow has yet to be elucidated.
Further investigation, in particular involving normal synovial tissue and arthritic cases with clearly defined disease stages, has to be performed to clarify that question.
References
Phillips PE . Viral arthritis. Curr Opin Rheumatol 1997; 9: 337–344.
Krause A, Kamradt T, Burmester GR . Potential infectious agents in the induction of arthritides. Curr Opin Rheumatol 1996; 8: 203–209.
Takeda T, Mizugaki Y, Matsubara L, Imai S, Koike T, Takada K . Lytic Epstein-Barr virus infection in the synovial tissue of patients with rheumatoid arthritis. Arthritis Rheum 2000; 43: 1218–1225.
Alspaugh MA, Tan EM . Serum antibody in rheumatoid arthritis reactive with cell associated antigen: demonstration by precipitation and immunofluorescence. Arthritis Rheum 1976; 19: 711–719.
Scully TB, Walker PJ, Moss DJ, Pope JH . Identification of multiple Epstein-Barr virus-induced nuclear antigens with sera from patients with rheumatoid arthritis. J Virol 1984; 52: 88–93.
Takahashi Y, Murai C, Shibata S, Munakata Y, Ishii T, Ishii K, et al. Human parvovirus B19 as a causative agent for rheumatoid arthritis. Proc Natl Acad Sci U S A 1998; 95: 8227–8232.
Saal JG, Steidle M, Einsele H, Muller CA, Fritz P, Zacher J . Persistence of B19 parvovirus in synovial membranes of patients with rheumatoid arthritis. Rheumatol Int 1992; 12: 147–151.
Stahl H-D, Hubner B, Seidl B, Liebert UG, van der Heijden IM, Wilbrink B, et al. Detection of multiple viral DNA species in synovial tissue and fluid of patients with early arthritis. Ann Rheum Dis 2000; 59: 342–346.
Newkirk MM, Watanabe DKN, Leclerc J, Lambert N, Shiroky JB . Detection of cytomegalovirus, Epstein-Barr virus and herpes virus-6 in patients with rheumatoid arthritis with or without Sjögren’s syndrome. Br J Rheumatol 1994; 33: 317–322.
Nikkari S, Luukkainen R, Mottonen T, Meurman O, Hannonen P, Skurnik M, et al. Does parvovirus B19 have a role in rheumatoid arthritis? Ann Rheum Dis 1994; 53: 106–111.
Speyer I, Breedveld FC, Dijkmans BAC . Human parvovirus B19 infection is not followed by inflammatory joint disease during long term follow-up. A retrospective study of 54 patients. Clin Exp Rheumatol 1998; 16: 576–578.
Anderson MJ, Lewis E, Kidds IM, Hall SM, Cohen BJ . An outbreak of erythema infectiosum associated with human parvovirus infection. J Hyg (Lond) 1984; 93: 85–93.
Anderson LJ, Tsou C, Parker RA, Chorba TL, Wulff H, Tattersall P, et al. Detection of antibodies and antigens of human parvovirus B19 by enzyme-linked immunosorbent assay. J Clin Microbiol 1986; 24: 522–526.
Cohen BJ, Buckley MM . The prevalence of antibody to human parvovirus B19 in England and Wales. J Med Microbiol 1988; 25: 151–153.
Brown KE, Anderson SM, Young NS . Erythrocyte P. antigen: cellular receptor for B19 parvovirus. Science 1993; 262: 114–117.
Srivastava A, Lu L . Replication of B19 parvovirus in highly enriched hematopoietic progenitor cells from normal bone marrow. J Virol 1988; 62: 3059–3063.
Young N . Hematologic and hematopoietic consequences of B19 parvovirus infection. Semin Hematol 1988; 25: 159–172.
Pattison JR, Jones SE, Hodgson J, Davis LR, White JM, Stroud CE, et al. Parvovirus infections and hypoplastic crisis in sickle-cell anaemia. Lancet 1981; 1: 664–665.
Pillet S, Morinet F . Parvovirus and erythroid cells. Pathol Biol (Paris) 2002; 50: 349–356.
Tolfvenstam T, Papadogiannakis N, Norbeck O, Petersson K, Broliden K . Frequency of human parvovirus B19 infection in intrauterine fetal death. Lancet 2001; 357: 1494–1497.
Miller E, Fairley CK, Cohen BJ, Seng C . Immediate and long term outcome of human parvovirus B19 infection in pregnancy. Br J Obstet Gynaecol 1998; 105: 174–178.
Mehraein Y, Rehder H, Draeger HG, Froster-Iskenius UG, Schwinger E, Holzgreve W . Die diagnostik fetaler virusinfektionen durch in-situ-hybridisierung. Geburtshilfe Frauenheilkd 1991; 51: 984–989.
Reid DM, Reid TMS, Brown T, Rennie RAN, Eastmond CJ . Human parvovirus-associated arthritis: a clinical and laboratory description. Lancet 1985; 1: 422–425.
White DG, Woolf AD, Mortimer PP, Cohen BJ, Blake DR, Bacon PA . Human parvovirus arthropathy. Lancet 1985; 1: 419–421.
Söderlund M, von Essen R, Haapasaari J, Kiistala U, Kiviluoto O, Hedman K . Persistence of parvovirus B19 DNA in synovial membranes of young patients with and without chronic arthropathy. Lancet 1997; 349: 1063–1065.
Hokynar K, Brunstein J, Söderlund-Venermo M, Kiviluoto O, Partio EK, Konttinen Y, et al. Integrity and full coding sequence of B19 virus DNA persisting in human synovial tissue. J Gen Virol 2000; 81: 1017–1025.
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 1988; 31: 315–324.
Cotmore SF, McKie VC, Anderson LJ, Astell CR, Tattersall P . Identification of the major structural and nonstructural proteins encoded by human parvovirus B19 and mapping of their genes by procaryotic expression of isolated genomic fragments. J Virol 1986; 60: 548–557.
Shade RO, Blundell MC, Cotmore SF, Tattersall P, Astell CR . Nucleotide sequence and genome organization of human parvovirus B19 isolated from the serum of a child during aplastic crisis. J Virol 1986; 58: 921–936.
Cooling LLW, Koerner TAW, Naides SJ . Multiple glycosphingolipids determine the tissue tropism of parvovirus B19. J Infect Dis 1995; 172: 1198–1205.
Imai Y, Sato T, Yamakawa M, Kasjima T, Suda A, Watanabe Y . A morphological and immunohistochemical study of lymphoid germ centers in synovial and lymph node tissues from rheumatoid arthritis patients with special reference to complement components and their receptors. Acta Pathol Jpn 1989; 39: 127–134.
Modrow S, Dorsch S . Antibody responses in parvovirus B19 infected patients. Pathol Biol (Paris) 2002; 50: 326–331.
Kurtzman GJ, Ozawa K, Cohen B, Hanson G, Oseas R, Young NS . Chronic bone marrow failure due to persistent B19 parvovirus infection. N Engl J Med 1987; 317: 287–294.
Lundquist A, Tolfvenstam T, Brytting M, Stolt CM, Hedman K, Broliden K . Prevalence of parvovirus B19 DNA in bone marrow of patients with haematological disorders. Scand J Infect Dis 1999; 31: 119–122.
Cassinotti P, Siegl G, Michel BA, Bruhlmann P . Presence and significance of human parvovirus B19 DNA in synovial membranes and bone marrow from patients with arthritis of unknown origin. J Med Virol 1998; 56: 199–204.
Acknowledgements
The authors thank Dr. Ina Müller-Molaian and Gertrud Walter, Department of Pathology, Saarland University, Homburg, Germany, for support in constituting the study collective and for excellent technical assistance. Furthermore, we are grateful to Dr. Bodo Fischer from Dade-Behring, Marburg, Germany, for supply with B19-VP1/VP2-antibody as well as B19-VP1/VP2-antigen.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mehraein, Y., Lennerz, C., Ehlhardt, S. et al. Detection of Parvovirus B19 Capsid Proteins in Lymphocytic Cells in Synovial Tissue of Autoimmune Chronic Arthritis. Mod Pathol 16, 811–817 (2003). https://doi.org/10.1097/01.MP.0000083145.68333.9B
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1097/01.MP.0000083145.68333.9B
Keywords
This article is cited by
-
Human parvovirus B19 infection in malignant and benign tissue specimens of different head and neck anatomical subsites
Infectious Agents and Cancer (2023)
-
Infections and the risk of psoriatic arthritis among psoriasis patients: a systematic review
Rheumatology International (2018)
-
Low levels of antibodies against common viruses associate with anti-citrullinated protein antibody-positive rheumatoid arthritis; implications for disease aetiology
Arthritis Research & Therapy (2017)
-
Extinct type of human parvovirus B19 persists in tonsillar B cells
Nature Communications (2017)
-
A high prevalence of parvovirus B19 DNA in patients with psoriasis
Archives of Dermatological Research (2006)