Abstract
Mesothelin is a cell surface antigen of unknown function that is strongly expressed in mesothelial cells. Although it was reported in 1992 that immunostaining with the K1 anti-mesothelin antibody could be very useful in distinguishing between epithelioid mesotheliomas and pulmonary adenocarcinomas, no further studies have been published on the value of this marker in the diagnosis of mesotheliomas. To determine whether mesothelin can assist in discriminating epithelioid mesotheliomas from lung adenocarcinomas or from other carcinomas metastatic to the serosal membranes, 55 mesotheliomas (44 epithelioid, 3 biphasic, and 8 sarcomatoid), 48 carcinomas of the lung (31 adenocarcinomas, 17 squamous carcinomas), and 86 nonpulmonary adenocarcinomas (14 ovary, 5 peritoneum, 9 endometrium, 11 pancreas, 4 stomach, 16 colon, 12 breast, 9 kidney, 4 thyroid, and 2 prostate) were investigated for mesothelin expression using the recently available 5B2 anti-mesothelin monoclonal antibody. Reactivity was obtained in all 44 (100%) of the epithelioid mesotheliomas, 12 (39%) of the lung adenocarcinomas, and 42 (49%) of the nonpulmonary adenocarcinomas (14 [100%] ovary; 5 [100%] peritoneum; 6 [67%] endometrium; 10 [91%] pancreas; 2 [50%] stomach; 5 [31%] colon; and in none [0] of the breast, kidney, thyroid, or prostate). Three (18%) of the squamous carcinomas of the lung, but none of the sarcomatoid mesotheliomas, exhibited positivity for this marker, nor was any reactivity seen in the spindle cell component of the biphasic mesotheliomas. It is concluded that despite the low specificity of mesothelin for discriminating between epithelioid mesotheliomas and adenocarcinomas, immunostaining for this marker may have some utility in those instances in which the results obtained with the standard panel of immunohistochemical markers used for the diagnosis of mesotheliomas are equivocal. Because mesothelin is a highly sensitive positive marker for epithelioid mesotheliomas, a negative staining for this marker is an indication against such a diagnosis; however, because of its limited utility, it is not recommended for inclusion in the standard panel of immunohistochemical markers used in the distinction between mesotheliomas and adenocarcinomas.
Similar content being viewed by others
INTRODUCTION
A well-known problem in surgical pathology is the distinction of pleural mesothelioma from peripheral pulmonary adenocarcinoma involving the pleura or from a metastatic adenocarcinoma originating from a distant organ and presenting as a tumor of unknown origin. This differential diagnosis, however, has been greatly facilitated by the use of immunohistochemical markers. Because a specific marker for mesothelioma has not yet been identified, the immunohistochemical diagnosis depends on the use of panels of markers that until recently were composed primarily of antibodies that frequently reacted with adenocarcinomas but not with mesotheliomas (1, 2, 3, 4). Since the mid-1990s, however, a large number of antibodies that commonly react with mesotheliomas, but not with adenocarcinomas, have become available (3, 5, 6, 7, 8, 9, 10, 11, 12).
In 1992, using the K1 anti-mesothelin monoclonal antibody on frozen tissue specimens, Chang et al. (13) reported mesothelin expression in all of 15 epithelioid mesotheliomas but in none of 23 pulmonary adenocarcinomas. On the basis of this observation, those investigators concluded that mesothelin immunostaining could be useful in discriminating between these two malignancies. Despite such promising results, no other investigations on the value of mesothelin in the diagnosis of mesothelioma have been published, and the Chang et al. (13) observation has remained unconfirmed. An anti-mesothelin monoclonal antibody capable of reacting on routinely fixed and processed specimens has recently become commercially available. The purpose of this study is to investigate the value of this antibody in the diagnosis of mesothelioma.
MATERIALS AND METHODS
The material used in this study was obtained from the files of the Department of Pathology at the University of Texas M.D. Anderson Cancer Center and is listed in Table 1. It consists of 55 unequivocal mesotheliomas (44 epithelioid, 8 sarcomatoid, 3 biphasic), 48 primary lung carcinomas (31 adenocarcinomas, 17 squamous carcinomas), and 86 nonpulmonary carcinomas, which consisted of 14 nonmucinous carcinomas of the ovary (11 serous and 3 endometrioid), 5 primary peritoneal serous carcinomas, 9 endometrial adenocarcinomas, 11 pancreatic ductal adenocarcinomas, 16 colonic adenocarcinomas, 4 gastric adenocarcinomas, 12 breast carcinomas, 9 renal cell carcinomas, 4 papillary thyroid carcinomas, and 2 prostatic adenocarcinomas. The lung adenocarcinomas were diagnosed using the World Health Organization’s criteria (14). Fifteen were acinar, 8 were papillary, 6 were solid with mucin production, and 2 were bronchioloalveolar. For all of the mesotheliomas, the diagnosis was made using currently accepted histologic criteria on hematoxylin-and-eosin stained sections combined with immunohistochemical, ultrastructural, and clinical features. All of these cases showed strong reactivity for calretinin and cytokeratin 5/6 but were negative for carcinoembryonic antigen, B72.3, and MOC-31, findings that are considered to be supportive of the diagnosis of mesothelioma (15). Immunoperoxidase studies were performed on formalin-fixed, paraffin-embedded tissue sections using the avidin-biotin-peroxidase complex (ABC) method in a DAKO AutoStainer (Carpinteria, CA). The primary antibody used was the 5B2 anti-mesothelin monoclonal antibody (Novocastra, Newcastle-on-Tyne, UK; 1:30 dilution). The immunoperoxidase staining was done using the LSAB2 peroxidase kit (DAKO). To enhance the immunostaining, a heat epitope retrieval procedure was performed using a Black-and-Decker (Shelton, CT) vegetable steamer. Briefly, deparaffinized sections were placed in a thermoresistant container filled with a 10:1 solution of Tris-EDTA buffer, pH 8.0, steamed for 45 minutes, then cooled for 20 minutes before immunostaining. The antigen–antibody immunoreaction was visualized using 3,3′-diaminobenzidene tetrahydrochloride as chromogen. To evaluate the specificity of the immunoreaction, known positive and negative tissue sections were used as controls. In addition, sections of the tumors were stained with the universal negative control, mouse (DAKO). The grading of the immunostaining was performed on a sliding scale of 1+ to 4+ according to the percentage of reactive cells (trace = <1%; 1+ = 1–25%; 2+ = 26–50%; 3+ = 51–75%; 4+ = >76%).
RESULTS
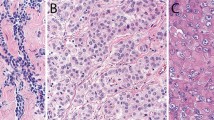
All 44 (100%) of the epithelioid mesotheliomas exhibited reactivity for mesothelin on the cell membranes. This was particularly strong, thick, and uniform along the apical surface (Fig. 1, A–B). A similar staining pattern was present in the epithelioid component of the three biphasic tumors. No reactivity was seen in any of the eight sarcomatoid mesotheliomas or in the spindle cell component of the biphasic tumors.
Twelve (39%) of the 31 lung adenocarcinomas exhibited mesothelin reactivity. In 6 of the tumors, the staining was primarily along the apical surface of the cells; in 5, it was mixed membranous and cytoplasmic; and in 1, it was exclusively cytoplasmic (Fig. 2, A–B). Among the nonpulmonary carcinomas, reactivity was seen in 14 of 14 originating in the ovary, 5 of 5 in the peritoneum, 10 of 11 in the pancreas, 6 of 9 in the endometrium, 5 of 16 in the colon, and 2 of 4 in the stomach. The staining in the ovarian carcinomas and the primary peritoneal serous carcinomas was usually strong and exhibited a similar pattern to that seen in the mesotheliomas. In the papillary areas, the reactivity mainly occurred along the apical surfaces of the neoplastic cells, whereas in the solid areas, the entire cell membrane was often involved (Fig. 3, A–B). In the other adenocarcinomas, although the staining was primarily apical, some cells also presented strong cytoplasmic positivity (Fig. 4). Only 3 of the 17 squamous carcinomas of the lung were mesothelin positive. In all of these cases, the staining was membranous and occurred in small, focal areas of the tumor (5 to 10% of the neoplastic cells). The immunohistochemical results are summarized in Table 1.
A, papillary serous carcinoma showing a thick, continuous mesothelin reactivity along the apical surface of the cells. B, high-grade serous carcinoma showing that in the papillary, areas the reaction occurred exclusively along the apical surface of the cells, whereas in the solid areas, it involved the entire cell membrane.
DISCUSSION
Mesothelin is a differentiation antigen that was originally described as the antigenic target of the K1 monoclonal antibody (16, 17). This antibody was generated using the OVCAR-3 ovarian carcinoma cell line. It is now known that mesothelin is a 40-kDa glycosyl-phosphatidylinositol (GPI)-linked glycoprotein that is synthesized as a 69 kDa precursor that is enzymatically processed into an N-terminal–secreted form of molecular mass 30 kDa and a GPI-linked membrane-bound form of 40 kDa (18). The secreted form is identical to the megakaryocyte-potentiating factor (19). It is, however, the GPI-linked membrane form that has generated the most interest as a potential immunohistochemical marker in tumor diagnosis (20).
Because mesothelin is strongly expressed in normal mesothelial cells and in mesotheliomas, Chang et al. (13) investigated the potential use of this protein as an immunohistochemical marker for distinguishing between epithelioid mesotheliomas and pulmonary adenocarcinomas. Those investigators reported strong mesothelin expression in all of the epithelioid mesotheliomas investigated using the K1 anti-mesothelin antibody on frozen tissue specimens, whereas all of the pulmonary adenocarcinomas were negative for this marker. On the basis of these results, it was concluded that the K1 antibody was a useful positive mesothelial marker for discriminating between these two malignancies. These results are in contrast to those obtained in the present investigation using the 5B2 anti-mesothelin monoclonal antibody that has only recently become commercially available. In this study, even though mesothelin expression was demonstrated in all 44 (100%) epithelioid mesotheliomas, it was also found to occur in 39% of the lung adenocarcinomas. Some differences in the staining pattern were noticed, however. Although the reactivity in the mesotheliomas was more often membranous, strong, and diffuse, in the lung adenocarcinomas it was usually focal, sometimes mixed membranous and cytoplasmic, or predominantly cytoplasmic. Despite these differences, the fact that more than one third of the lung adenocarcinomas were mesothelin positive and 12 mesotheliomas exhibited focal reactivity (1+ or 2+) significantly limit the value of mesothelin immunostaining in discriminating between these malignancies. Only one of the four sarcomatoid mesotheliomas stained by Chang et al. (13) with the K1 anti-mesothelin antibody was reported as being positive in only 20% of the neoplastic cells. In the present study, all eight sarcomatoid mesotheliomas, as well as the spindle cell component of the three biphasic mesotheliomas, were mesothelin negative. This finding indicates that mesothelin immunostaining has no utility in the diagnosis of sarcomatoid mesotheliomas.
A great variation of mesothelin expression was found among the nonpulmonary adenocarcinomas investigated in the present study. All 14 non-mucinous ovarian carcinomas and all 5 peritoneal serous carcinomas investigated were strongly mesothelin positive. Although the percentage of ovarian carcinomas exhibiting mesothelin expression is higher than was obtained by Chang et al. (17), who reported mesothelin expression in 10 of 15 (66%) nonmucinous ovarian carcinomas using the K1 antibody, it confirms that mesothelin immunostaining has no utility for distinguishing between epithelioid mesotheliomas and primary peritoneal serous carcinomas or metastatic ovarian carcinomas to the serosal membranes.
Besides ovarian carcinomas, adenocarcinomas of the pancreas were another malignancy that was found to frequently express mesothelin in the present study. Ten of the 11 pancreatic adenocarcinomas investigated were mesothelin positive, and in most of these cases, the staining was strong and diffuse. These results are similar to those reported by Argani et al. (20) who, using the same anti-mesothelin antibody employed in the present investigation, demonstrated mesothelin expression in all 60 of their pancreatic adenocarcinomas. In that study, the staining was reported to be focal (<25% of the tumor cells) in 10 cases (17%), diffuse (>75% of the tumor cells) in 18 cases (30%), and in the remaining 32 cases, reactivity was seen in 25 to 75% of the neoplastic cells. Also in that investigation, the normal pancreas in 52 of the 60 cases was reported as being essentially nonreactive, whereas in the remaining 8, a faint “blush” staining in scattered pancreatic ducts was observed. This observation was considered to be important because it suggested that strong mesothelin immunostaining may assist in separating neoplastic from non-neoplastic ductal epithelial cells in small biopsies and cytologic specimens. In addition to adenocarcinomas of the lung, pancreas, and ovary, those originating in the endometrium, stomach, and colon were also found to express mesothelin. No reactivity, however, was seen in any of the adenocarcinomas of the breast, kidney, thyroid, or prostate.
Only 3 (18%) of 17 squamous carcinomas of the lung in the present investigation exhibited mesothelin reactivity, and the staining in these cases was limited to small areas of the tumor. This finding is in contrast to that of Chang et al. (21), who were able to demonstrate mesothelin expression with the K1 antibody in 8 (80%) of 10 squamous carcinomas of the lung. The staining obtained in those cases was usually strong, in contrast to the results seen in the present study, in which the reactivity was limited to only 5 to 10% of the neoplastic cells.
In conclusion, the results in the present study confirm previous reports that indicate that mesothelin is strongly expressed in epithelioid mesotheliomas and ovarian carcinomas. In contrast to previous reports, however, this study has found that mesothelin can also be expressed in lung adenocarcinomas, thus limiting the practical value of this marker for discriminating between epithelioid mesotheliomas and pulmonary adenocarcinomas. Although mesothelin is not recommended for inclusion in the standard immunohistochemical panel used to assist in distinguishing between these malignancies (15), it may have some utility in those cases in which the results are equivocal. Because of the common and strong mesothelin expression in epithelioid mesotheliomas, a negative staining for this marker can be regarded as a strong indication against such a diagnosis.
References
Brown RW, Campagna LB, Dunn JK, Cagle PT . Immunohistochemical identification of tumor markers in metastatic adenocarcinoma: a diagnostic adjunct in the determination of primary site. Am J Clin Pathol 1997; 107: 12–19.
Ordóñez NG . The immunohistochemical diagnosis of mesothelioma. Differentiation of mesothelioma and lung adenocarcinoma. Am J Surg Pathol 1989; 13: 276–291.
Ordóñez NG . Value of antibodies 44–3A6, SM3, HBME-1 and thrombomodulin in differentiating epithelial pleural mesothelioma from lung adenocarcinoma: a comparative study with other commonly used antibodies. Am J Surg Pathol 1997; 21: 1399–1408.
Riera JR, Astengo-Osuna C, Longmate JA, Battifora H . The immunohistochemical diagnostic panel for epithelial mesothelioma. A reevaluation after heat-induced epitope retrieval. Am J Surg Pathol 1997; 21: 1409–1419.
Amin KM, Litzky LA, Smythe WR, Mooney AM, Morris JM, Mews DJY, et al. Wilms’ tumor 1 susceptibility (WT1) gene products are selectively expressed in malignant mesothelioma. Am J Pathol 1995; 146: 344–356.
Clover J, Oates J, Edwards C . Anti-cytokeratin 5/6. A positive marker for epithelioid mesothelioma. Histopathology 1997; 31: 140–143.
Collins CL, Ordóñez NG, Schaefer R, Cook CD, Xie S-S, Granger J, et al. Thrombomodulin expression in malignant pleural mesothelioma and pulmonary adenocarcinoma. Am J Pathol 1992; 141: 827–833.
Doglioni C, Dei Tos AP, Laurino L, Iuzzolino P, Chiarelli C, Celio MR, et al. Calretinin. A novel immunocytochemical marker for mesothelioma. Am J Surg Pathol 1996; 20: 1037–1046.
Ordóñez NG . In search of a positive immunohistochemical marker for mesothelioma: an update. Adv Anat Pathol 1998; 5: 53–60.
Ordóñez NG . Value of thyroid transcription factor-1, E-cadherin, BG8, WT1, and CD44S immunostaining in distinguishing epithelial pleural mesothelioma from pulmonary and nonpulmonary adenocarcinoma. Am J Surg Pathol 2000; 24: 598–606.
Carella R, Deleonardi G, D’Errico A, Salerno A, Egarter-Vigl E, Seebacher C, et al. Immunohistochemical panels for differentiating epithelial malignant mesothelioma from lung adenocarcinoma: a study with logistic regression analysis. Am J Surg Pathol 2001; 25: 43–50.
Ordóñez NG . Value of calretinin immunostaining in differentiating epithelial mesothelioma from lung adenocarcinoma. Mod Pathol 1998; 11: 929–933.
Chang K, Pai LH, Pass H, Pogrebniak HW, Tsao M-S, Pastan I, et al. Monoclonal antibody K1 reacts with epithelial mesothelioma but not with lung adenocarcinoma. Am J Surg Pathol 1992; 16: 259–268.
Travis WD, Colby TV, Corrin B, et al. World Health Organization international histological classification of tumors: histologic typing of lung and pleural tumors. Berlin: Springer; 1999.
Ordóñez NG . The immunohistochemical diagnosis of epithelioid mesotheliomas: old markers, new markers—a critical review. Hum Pathol (in press).
Chang K, Pai LH, Batra JK, Pastan I, Willingham MC . Characterization of the antigen (CAK1) recognized by monoclonal antibody K1 present on ovarian cancers and normal mesothelium. Cancer Res 1992; 52: 181–186.
Chang K, Pastan I, Willingham MC . Isolation and characterization of a monoclonal antibody, K1, reactive with ovarian cancers and normal mesothelium. Int J Cancer 1992; 50: 373–381.
Chang K, Pastan I . Molecular cloning of mesothelin, a differentiation antigen present on mesothelium, mesotheliomas, and ovarian cancers. Proc Natl Acad Sci U S A 1996; 93: 136–140.
Kojima T, Oh-eda M, Hattori K, Taniguchi Y, Tamura M, Ochi N, Yamaguchi N . Molecular cloning and expression of megakaryocyte potentiating factor cDNA. J Biol Chem 1995; 270: 21984–21990.
Argani P, Iacobuzio-Donahue C, Ryu B, Rosty C, Goggins M, Wilentz RE, et al. Mesothelin is overexpressed in the vast majority of ductal adenocarcinomas of the pancreas: identification of a new pancreatic cancer marker by serial analysis of gene expression (SAGE). Clin Cancer Res 2001; 7: 3862–3868.
Chang K, Pastan I, Willingham MC . Frequent expression of the tumor antigen CAK1 in squamous-cell carcinomas. Int J Cancer 1992; 51: 548–554.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Ordóñez, N. Value of Mesothelin Immunostaining in the Diagnosis of Mesothelioma. Mod Pathol 16, 192–197 (2003). https://doi.org/10.1097/01.MP.0000056981.16578.C3
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1097/01.MP.0000056981.16578.C3
Keywords
This article is cited by
-
Mesothelin-targeted CAR-T therapy combined with irinotecan for the treatment of solid cancer
Journal of Cancer Research and Clinical Oncology (2023)
-
The Evolving Therapeutic Landscape for Malignant Pleural Mesothelioma
Current Oncology Reports (2022)
-
Possible reversibility between epithelioid and sarcomatoid types of mesothelioma is independent of ERC/mesothelin expression
Respiratory Research (2020)
-
Mesothelin expression has prognostic value in stage ΙΙ/ΙΙΙ colorectal cancer
Virchows Archiv (2019)
-
Prospects of chimeric antigen receptor T cell therapy in ovarian cancer
Medical Oncology (2018)