Abstract
E-cadherin/catenin complex regulates cellular adhesion and motility and is believed to function as an invasion suppressor system. In a number of cancers, abnormal and reduced expression of E-cadherin/catenin complex is associated with tumor invasion and metastasis. Prolactinomas show frequent invasion on the surrounding structures, despite their histologically benign nature. Furthermore, gender-based differences in endocrine and surgical findings are found in patients with prolactinoma. To understand biological factors governing prolactinoma behavior, this study analyzed the expression of E-cadherin; α-, β-, and γ-catenins; p120; and cell proliferation marker MIB-1 labeling index in 13 invasive tumors (9 in men, 4 in women), 26 noninvasive tumors (4 in men, 22 in women), and 8 normal anterior pituitaries by immunohistochemistry. Immunostaining of E-cadherin; α-, β-, and γ-catenins; and p120 showed a membranous pattern of reactivity and generally stronger in normal pituitaries than in prolactinomas. Expression of E-cadherin and β-catenin was significantly lower in invasive than in noninvasive prolactinomas (P <.002 and P <.005, respectively), and reduced expression of E-cadherin and β-catenin was more frequent in invasive than in noninvasive prolactinomas (P <.001 and P <.05, respectively); in contrast, γ-catenin expression showed higher in invasive than in noninvasive prolactinomas (P <.05). Expression of E-cadherin was significantly lower in macroprolactinomas than in microprolactinomas (P <.01), and decreased expression of E-cadherin and β-catenin predicted high MIB-1 expression (P <.05). Moreover, the expression of E-cadherin and β-catenin was significantly lower in macroprolactinomas in men than in those in women (P <.01 and P <.02, respectively). No statistical correlations were observed between expression of α-catenin, p120, and clinicopathologic features. In conclusion, the reduction of E-cadherin and β-catenin expression was related to invasiveness and proliferative status of prolactinomas and correlated with the more aggressive behavior of prolactinomas in men compared with in women.
Similar content being viewed by others
INTRODUCTION
Prolactinomas are the most common type of pituitary tumors (1, 2, 3). Despite their histologically benign nature, PRL-secreting pituitary adenomas show frequent invasion of the surrounding structures such as the sphenoid sinus, the cavernous sinus, and, in some cases, the brain. The biological factors governing their invasive tendencies and reliable markers for predicting tumors’ behavior remain poorly understood. Moreover, gender-specific differences in tumor size and the biological behavior of tumors are found at clinical presentation of prolactinoma, such that macro and invasive tumors are more commonly found in men than in women. The molecular bases and mechanisms for these differences remain to be determined (4).
Tumor cell invasion is a multistep process requiring complex alterations in adhesive interactions. Detachment of tumor cells from the primary lesion is considered to be an important step in the invasion process. One of the molecules implicated in the progress of tumor invasion is E-cadherin (5). It plays a significant role in the establishment of cell-cell interaction within epithelial cells of neoplasm as well as normal tissue (6, 7). Numerous reports have discussed the association between reduction or loss of E-cadherin expression by tumor cells and the histological differentiation, invasion, metastasis, recurrence, and prognosis of tumors of epithelial origin (8). The functions of E-cadherin are mediated through its cytoplasmic linkage to the actin cytoskeleton via certain cytoplasmic plaque proteins known as the catenins (α-, β-, and γ-catenins; 9, 10). p120, binding to E-cadherin and regulating its function, is necessary to enable the adequate function of intercellular adhesion (11). Regarding pituitary tumors, a few reports have studied the expression of E-cadherin, α-, β-, and γ-catenins (12, 13, 14). There were few available data regarding the relationship between these expressions and clinicopathological findings, and to our knowledge, expression of p120 has not been reported in prolactinoma.
The reasons for gender-specific differences in the biologic behavior of prolactinomas are unknown. A delay in seeking medical attention explains why tumors are larger in men (15, 16). In contrast, Delgrange et al. reported that the predominance of large prolactinomas in men is due to high frequency of rapidly growing tumors, which are often invasive and frequently bromocriptine resistant (4).
To investigate the role of cell adhesion molecules in the invasion process and the proliferation of prolactinomas and to explore the biological reasons for the gender-based differences in clinical findings, we examined expression of E-cadherin and of α-, β-, and γ-catenins and p120 in 39 prolactinomas as well as in 8 specimens of normal pituitary gland by immunohistochemistry. The relations between their expression and clinicopathological findings and proliferation marker (MIB-1) were evaluated.
MATERIALS AND METHODS
Patients
Thirty-nine prolactinomas were obtained during operative treatment at Tokushima University Hospital (Tokushima, Japan) and Toranomon Hospital (Tokyo, Japan) between 1988 and 2001. Histological examination, immunohistochemistry for anterior pituitary hormones, and the clinical, biological, and radiological data were used to fully characterize each tumor. A diagnosis of pure prolactinoma in all of the patients was confirmed by immunohistochemistry. The patients with clinical or biological evidence of pituitary mixed tumors were excluded from the study. This study included 13 patients with invasive tumors (9 in men, 4 in women) and 26 patients with noninvasive tumors (4 in men, 22 in women); 11 of them (2 with invasive tumor, 9 with noninvasive tumor) were treated with dopamine agonist drugs as preoperative treatment. Tumor invasiveness was defined on the basis of the modified Hardy criteria. Eight specimens of normal anterior pituitary gland obtained at autopsy were also studied. The tissues had been fixed in 4% buffered formalin, dehydrated, and embedded in paraffin.
Immunohistochemistry
E-cadherin; α-, β- and γ-catenins; p120; and Ki-67 immunostains based on the labeled streptavidin biotin method were performed on 5-μm sections from representative blocks of paraffin-embedded tissues used for pathology diagnosis. Pertinent details regarding antibodies and staining procedure are summarized in Table 1.
The applications of these antibodies on formalin-fixed, paraffin-embedded material and their specificity have been described (4, 17, 18, 19, 20, 21). Sections incubated in PBS without the primary antibody served as negative controls. For positive controls, normal epidermis of skin tissues known to be positive for E-cadherin, catenins, and p120 were used. All slides were assessed under a light microscope by two independent observers (CCL and TS) without knowledge of the clinical parameters. Positive expression of E-cadherin, catenins, and p120 was defined as exclusively membranous staining, as seen in the normal epithelial cell of the epidermis. Both the intensity of staining and the percentage of positive tumor cells of E-cadherin, catenins, and p120 in each specimen were considered in semiquantitative assessment. The distribution of positive staining in the tumor was graded on the five-tier scoring system (−, no staining; 1+, 1–20%; 2+, 20–50%; 3+, 50–80%; and 4+, >80%). The intensity was subjectively graded as weak, moderate, or intense. The MIB-1 labeling index was determined by counting the number of positive cells in a total of 1000 tumor cells observed in several representative high-power fields (400 ×).
Statistical Analysis
We used StatView J-4.5 software to perform statistical analysis. Both the Mann-Whitney U test and chi-square test were performed to determine the significance of associations between different variables. The level of statistical significance was P <.05.
RESULTS
Clinical Data
As indicated in Table 2, the mean age at operation was 43 ± 4 years (range, 17–72 y) in 13 men and 30 ± 2 years (range, 17–65 y) in 26 women (P <.05). The mean pretreatment serum PRL level was significantly higher in men (3407 ± 1486 ng/mL; range, 114–20,000 ng/mL) than in women (206 ± 39 ng/mL; range, 50–900 ng/mL; P <.0001). The prevalence of macroadenomas that were >10 mm in diameter was significantly higher in male patients (100%) than in female patients (46%; P <.001). Similarly, invasive tumors were more common in male patients (69%) than in female patients (15%; P <.001). Giant prolactinomas, >40 mm in diameter, were only present in men (2 of 13). In addition, pretreatment serum PRL levels were higher in invasive adenomas (1840 ± 573 ng/mL; range, 80–6000 ng/mL) and macroadenomas (1871 ± 825 ng/mL; range, 50–20,000 ng/mL) than in noninvasive adenomas (989 ± 761 ng/mL; range, 50–20,000 ng/mL) and microadenomas (204 ± 68 ng/mL; range, 57–900 ng/mL; P <.005, P <.005, respectively).
Immunohistochemical Analysis of E-cadherin; α-, β-, and γ-Catenins; and p120
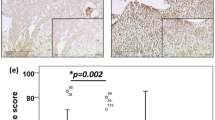
In 8 normal adenohypophyseal simples, E-cadherin; α-, β-, and γ-catenins; and p120 were expressed strongly on nearly entire hormone-producing cell-cell boundaries, without cytoplasmic and nuclear presence. In prolactinomas, positive immunostaining of E-cadherin; α-, β-, and γ-catenins; and p120 always showed a membranous pattern of reactivity without cytoplasmic and nuclear presence. The immunostaining results are illustrated in Figure 1.
A–D, noninvasive prolactinomas showing prominent membranous immunoreactivity for E-cadherin, α-catenin, β-catenin, and p120. F–I, in invasive prolactinomas, significant reduction of membranous staining of these cell adhesion molecules was shown. E and J, different from other cell adhesion molecules, γ-catenin generally showed the weakest membranous immunoreactivity; down-regulation of membranous staining of γ-catenin showing in noninvasive and invasive prolactinomas (original magnification, 50–100 ×).
All but seven prolactinomas showed a decrease in membranous E-cadherin expression; 14 (36%) tumors displayed a slightly reduced expression (3+), 8 (20.5%) demonstrated 2+ staining, 8 (20.5%) exhibited 1+ staining, and 2 (5%) showed no immunopositive cells (−). The expression of E-cadherin was significantly lower in invasive than in noninvasive prolactinomas (P <.002, Fig. 2A), lower in macroprolactinomas than microprolactinomas (P <.01, Fig. 2B); and lower in tumors in men than in those in women (P <.001, Fig. 2C). In addition, significantly reduced expression (scored as 1+, 2+, or no immunostaining) was observed more frequently in prolactinomas in invasive tumors (11 of 13, 85%), macroprolactinomas (15 of 25, 60%), and tumors men (11 of 13, 85%) than those in noninvasive tumors (7 of 26, 27%), microprolactinomas (3 of 14, 21%), and those in women (7 of 26, 27%; P <.001, P <.02, P <.001, respectively, Table 3). Moreover, the expression of E-cadherin was significantly lower in macroprolactinomas of men than in macroprolactinomas of women (P <.01, Fig. 2 D). In addition, significantly decreased expression of E-cadherin was observed more frequently in invasive tumors in men (9 of 9, 100%) than those in women (2 of 4, 50%; P <.05).
Membranous expression levels of E-cadherin; α-, β-, and γ-catenins; and p120 in prolactinomas. A, the expression levels of E-cadherin and β-catenin in invasive prolactinomas are significantly lower than those in noninvasive prolactinomas (1, P <.002; 2, P <.005). In contrast, the expression level of γ-catenin in noninvasive prolactinomas is significantly lower than that in invasive prolactinomas (3, P <.05). B, the expression level of E-cadherin in macroprolactinomas is significantly lower than that in microprolactinomas (1, P <.01). C, the expression levels of E-cadherin and β-catenin in prolactinomas in men are significantly lower than those in women (1, P<.001; 2, P <.01). D, the expression levels of E-cadherin and β-catenin in macroprolactinomas of men are significantly lower than those in macroprolactinomas of women (1, P <.01; 2, P <.02). E, the expression levels of E-cadherin and β-catenin in prolactinomas with high MIB-1 LB (>2%) are significantly lower than those in prolactinomas with low MIB-1 LB (<2%; 1, P <.05; 2, P <.05).
Reduced membranous expression of α-catenin was demonstrated in 27 of 39 prolactinomas: − in 2 cases (5%), 1+ in 5 cases (13%), 2+ in 9 cases (23%), and 3+ in 11 cases (28%). Immunohistochemical detection of β-catenin showed reduced membranous immunostaining in tumor cells as compared with in normal cells in 20 of 39 cases (51%): 1+ in 3 cases (8%), 2+ in 5 cases (13%), and 3+ in 12 cases (30%). The expression of β-catenin was significantly lower in invasive than in noninvasive prolactinomas (P <.005, Fig. 2A). Reduced expression of β-catenin was observed more frequently in invasive prolactinomas than in noninvasive prolactinomas (P <.05, Table 3). In addition, the expression of β-catenin showed significant reduction in tumors in men compared with in tumors in women (P <.01, Fig. 2C). In particular, the expression of β-catenin was significantly lower in macroprolactinomas of men than in macroprolactinomas of women (P <.02, Fig. 2D). γ-Catenin expression showed the weakest immunostaining. All prolactinomas demonstrated reduced membranous expression of γ-catenin: − in 17 cases (43%), 1+ in 16 cases (41%), 2+ in 5 cases (13%), and 3+ in 1 cases (3%). Different from E-cadherin and β-catenin, the expression of γ-catenin was statistically higher in invasive prolactinomas than in noninvasive prolactinomas (P <.05, Fig. 2A). Reduced membranous expression of p120 was demonstrated in 29 of 39 prolactinomas: − in 2 cases (5%), 1+ in 3 cases (8%), 2+ in 10 cases (26%), and 3+ in 14 cases (36%).
MIB-1 Labeling Index
Immunoreactivity for Ki-67 antigen was detected by positive nuclear staining for the antibody MIB-1. The higher MIB-1 LI (>2%) was observed more frequently in prolactinomas with invasion and of men (P <.02 and P <.02, respectively, Table 4). Moreover, decreased expression of E-cadherin and β-catenin was associated with high MIB-1 expression (P <.05 and P <.05, respectively, Fig. 2E). In addition, prolactinomas tended to exhibit higher MIB-1 LI in invasive cases, macroprolactinoma cases, and cases in men than in noninvasive cases, microprolactinoma cases, and cases in women (Table 4), although this trend did not reach statistical significance.
DISCUSSION
E-cadherin functions as an invasion-suppressor molecule, and its loss permits or enhances the invasion of adjacent normal tissues and the formation of distant metastasis (6, 22). It has been shown that alteration, either transient or permanent, in the expression of E-cadherin could allow some malignant cells more readily to detach from larger parental structures, invade locally, escape from the primary site, and, finally, metastasize to distant organs (23). Invasiveness is often associated with down-regulation of E-cadherin expression in many types of human cancer. However, invasiveness among pituitary adenomas continues to be a vaguely understood phenomenon, and the mechanism whereby pituitary tumors become invasive is poorly understood. Our study confirms a significant reduction of E-cadherin expression in invasive prolactinomas compared with in noninvasive prolactinomas. In addition, reduced expression of E-cadherin was observed more frequently in invasive prolactinomas than in noninvasive ones. These data suggest that the regulation of E-cadherin expression is related to aggressive behavior of prolactinomas and that its level of expression may act as a possible marker of prolactinomas that are more likely to invade. To our knowledge, the current study is the first report demonstrating a correlation between invasion and the reduced expression of E-cadherin in prolactinomas.
The matrix metalloproteinases (MMP)-2 and -9, both Type IV collagenases, are important for tumor invasion because they break down basement membrane, in particular by degrading collagen IV (24). The levels of MMP-2 and MMP-9 in human and rodent neoplasms directly correlate with invasion and metastasis, and specific inhibitors of MMPs were shown to inhibit tumor cell invasion (25). Thus, an enhanced activity of collagenase Type IV and a decreased expression of E-cadherin should promote tumor cell invasion and metastasis. This hypothesis has been confirmed in some published data (26, 27). Recently, Turner et al. (28) demonstrated that enhanced expression of MMP-9 is related to tumor invasiveness in prolactinomas. Herein our results showed significantly reduced expression of E-cadherin in invasive cases. Therefore, we need a prospective study to establish this hypothesis in the mechanism of prolactinoma invasion.
In vitro studies have shown that cell-cell adhesion is related strictly to proliferation and differentiation processes (29, 30, 31). In vivo, reduction of E-cadherin expression was accompanied by increased PCNA and/or MIB-1 expression in laryngeal and cutaneous squamous cell carcinomas (32, 33). In addition, decreased expression of E-cadherin correlated with tumor size in carcinomas of prostate, liver, and neuroendocrine tumors (21, 34, 35). In the current study, decreased expression of E-cadherin was associated with high MIB-1 expression and tumor size. Therefore, decreased cell-cell adhesion activity in prolactinomas may be related not only to the invasiveness but also to the proliferation and growth rate of tumor cells.
The mechanisms underlying the reduced or absent expression of E-cadherin in tumors are not fully understood. Recently, two possible mechanisms have been proposed. Inhibition of activity of trans-acting transcription factors that regulate E-cadherin expression has been suggested to be a mechanism responsible for the loss of E-cadherin expression in breast cancer cells (36). Alternatively, CpG island methylation in the E-cadherin promoter region has been proposed as another common mechanism of its inactivation in human tumors (37). To confirm that methylation of the E-cadherin promoter is responsible for the down-regulation of E-cadherin in prolactinomas, we examined the methylation status of E-cadherin promoter in eight prolactinomas with reduced E-cadherin protein expression by methylation-specific polymerase chain reaction. Hypermethylation of E-cadherin promoter was not detected in this small group (data not shown). Further molecular analysis remains to be performed.
Cells lacking α-catenin are unable to form stable adherens junctions despite normal expression of E-cadherin. Nevertheless, α-catenin does not bind directly to E-cadherin but interacts with it via β- and γ-catenins (38, 39). In our study, there was a likely association between reduced expression of α-catenin and invasive prolactinomas, although it was not statistically significant.
The β-catenin was originally described as an element of the E-cadherin/catenin complex. In our study, the β-catenin expression status was similar to that of E-cadherin. A significant association between reduction of β-catenin expression and invasiveness and proliferation of prolactinomas was demonstrated. On the other hand, β-catenin also has signaling roles in the cytoplasm and nucleus that are important in development and cancer; β-catenin is a key player in the Wnt/Wg signaling pathway and interacts directly with the tumor suppressor adenomatous polyposis coli (8, 40). In the study by Semba et al. (13), nuclear localization of β-catenin has been demonstrated in 21 of 37 (57%) pituitary adenomas, including one prolactinoma. However, the present study showed no nuclear staining in any prolactinomas. This finding is consistent with the results of Tziortzioti et al. (14).
The γ-catenin showed the weakest expression as compared with the other two catenins. In addition, absent expression of γ-catenin was observed more frequently in this series as compared with the case of the other two catenins. This finding is also consistent with the results of Tziortzioti et al. (14). Interestingly, increased expression of γ-catenin was associated with tumor invasion. The reason for these findings is unclear. Little is known about the role of γ-catenin in tumors, although γ-catenin binds to APC protein (41).
The p120 is a relatively new member of catenin-related protein to directly combine to E-cadherin and is likely to have additional roles in the nucleus (42). However, the precise function of p120 in human tumors has not been fully clarified. Decreased p120 has been reported in a variety of human tumors and in some cases is significantly linked to an aggressive tumor phenotype (17, 19, 42). In this study, reduction of p120 was demonstrated in 74% of prolactinomas, but no statistical correlation was detected between the expression of p120 and clinicopathologic features.
At the time of diagnosis, prolactinomas are larger in men than in women; there also may be differences in biological behavior of tumors between men and women. In this series, all of tumors in men were macroprolactinomas, including two giant tumors, and 69% of them showed invasive growth. These findings are in agreement with those in earlier reports (4, 15, 16). This preponderance of large or aggressive tumors in men has been thought due to a longer delay in diagnosis. This assumption supposes that micro- and macroadenomas represent two stages of the same disease and that a good correlation exists between duration of disease and tumor size (15, 16). On the other hand, recent data suggest that the predominance of large prolactinomas in men is due to a high frequency of rapidly growing tumors, which show frequent invasion (4). It is difficult to determine whether the preponderance of large or invasive tumors in men is due to specific characteristics of tumor cells rather than a delay in diagnosis or the opposite, partly because of their unclear duration of disease. To our knowledge, few studies have investigated the possible molecular bases and mechanisms for these differences. In our study, the expression of E-cadherin and β-catenin showed significant reduction in prolactinomas in men compared with those in women and particularly significant reduction in macroprolactinomas in men compared with those in women. In addition, significantly decreased expression of E-cadherin was observed more frequently in invasive tumors in men (9 of 9) than in women (2 of 4). Thus the regulation of E-cadherin/catenin complex seems to play a specific role in gender-based different behavior of prolactinomas. However, the expression of cell-cell adhesion molecules in microprolactinomas in men is unknown, and the group of invasive tumors in women in this limited series is too small. Further research in large series is required.
The MIB-1 LI has been investigated to determine its value in many types of neoplasm, but the usefulness of this marker as a predictor of the clinical behavior of pituitary tumors is still under evaluation. Based on our results, prolactinomas tended to exhibit higher MIB-1 LI in invasive cases, macroprolactinoma cases, or cases in men than in noninvasive cases, microprolactinoma cases, or cases in women. These findings are in general agreement with those in some published reports (4, 43). Furthermore, the higher MIB-1 LI (>2%) was observed more frequently in prolactinomas in invasive tumors and in tumors in men. So the invasive behavior of prolactinomas and a more aggressive course of the tumor in men may relate to higher cell proliferation.
This is the first report of the correlation between the expression of the cell adhesion molecules and behavior of prolactinomas. Our study not only confirms that decreased expression of E-cadherin and β-catenin is associated with tumor invasion but also shows that decreased cell-cell adhesion activity may be related to the tumor cell proliferation in prolactinomas. Moreover, the reduction of E-cadherin and β-catenin expression may play an important role in gender-based different behavior of prolactinomas, although the mechanism remains to be elucidated.
References
Horvath E, Scheithauer BW, Kovacs K, Lloyd RV . Regional neuropathology: hypothalamus and pituitary. In: Graham DI, Lantos Pl, editors. Greenfield’s neuropathology, 6th ed. Vol 1. London: Arnold Publishers; 1997: 1007–1094.
Kovacs K . Horvath E . Tumors of the pituitary gland. In: Hartmann WH, editors. Atlas of tumor pathology. Series 2, Fascicle 21. Washington, DC: Armed Forces Institute of Pathology; 1986. p. 1–264.
Scheithauer BW . Surgical pathology of the pituitary: the adenomas. Part 1. Pathol Annu 1984; 19 Pt 1: 317–374.
Delgrange E, Trouillas J, Maiter D, Donckier J, Tourniaire J . Sex-related difference in the growth of prolactinomas: a clinical and proliferation marker study. J Clin Endocrinol Metab 1997; 82: 2102–2107.
Vleminckx K, Vakaet L Jr, Mareel M, Fiers W, van Roy F . Genetic manipulation of E-cadherin expression by epithelial tumor cells reveals an invasion suppressor role. Cell 1991; 66: 107–119.
Takeichi M . Cadherins in cancer: implications for invasion and metastasis. Curr Opin Cell Biol 1993; 5: 806–811.
Shiozaki H, Oka H, Inoue M, Tamura S, Monden M . E-cadherin mediated adhesion system in cancer cells. Cancer 1996; 77: 1605–1613.
Van Aken E, De Wever O, Correiada Rocha AS, Mareel M . Defective E-cadherin/catenin complexes in human cancer. Virchows Arch 2001; 439: 725–751.
Ozawa M, Baribault H, Kemler R . The cytoplasmic domain of the cell adhesion molecule uvomorulin associates with three independent proteins structurally related in different species. EMBO J 1989; 8: 1711–1717.
Gumbiner BM, McCrea PD . Catenins as mediators of the cyto-plasmic functions of cadherins. J Cell Sci 1993; 17: 155–158.
Shibamoto S, Hayakawa M, Takeuchi K, Hori T, Miyazawa K, Kitamura N, et al. Association of p120, a tyrosine kinase substrate, with E-cadherin/catenin complexes. J Cell Biol 1995; 128: 949–957.
Kawamoto H, Mizoue T, Arita K, Tominaga A, Eguchi K, Kurisu K . Expression of epithelial cadherin and cavernous sinus invasion in human pituitary adenomas. J Neurooncol 1997; 34: 105–109.
Semba S, Han SY, Ikeda H, Horii A . Frequent nuclear accumulation of beta-catenin in pituitary adenoma. Cancer 2001; 91: 42–48.
Tziortzioti V, Ruebel KH, Kuroki T, Jin L, Scheithauer BW, Lloyd RV . Analysis of beta-catenin mutations and alpha-, beta-, and gamma-catenin expression in normal and neoplastic human pituitary tissues. Endocr Pathol 2001; 12: 125–136.
Spark RF, Wills CA, O’Reilly G, Ransil BJ, Bergland R . Hyperprolactinaemia in males with and without pituitary macroadenomas. Lancet 1982; 2: 129–132.
Gimenez-Roqueplo AP, Dupuy M, Delalande O, Visot A, Jedynak CP, Peillon F, et al. Prolactin microadenoma in men. Study of 14 cases [abstract]. Ann Med Interne (Paris) 1992; 143: 94–97.
Valizadeh A, Karayiannakis AJ, el-Hariry I, Kmiot W, Pignatelli M . Expression of E-cadherin-associated molecules (alpha-, beta-, and gamma-catenins and p120) in colorectal polyps. Am J Pathol 1997; 150: 1977–1984.
Hidaka N, Nagao T, Asoh A, Kondo Y, Nagao K . Expression of E-cadherin, alpha-catenin, beta-catenin, and gamma-catenin in bronchioloalveolar carcinoma and conventional pulmonary adenocarcinoma: an immunohistochemical study. Mod Pathol 1998; 11: 1039–1045.
Kallakury BV, Sheehan CE, Ross JS . Co-downregulation of cell adhesion proteins alpha- and beta-catenins, p120CTN, E-cadherin, and CD44 in prostatic adenocarcinomas. Hum Pathol 2001; 32: 849–855.
Sato H, Hasegawa T, Kanai Y, Tsutsumi Y, Osamura Y, Abe Y, et al. Expression of cadherins and their undercoat proteins (alpha-, beta-, and gamma-catenins and p120) and accumulation of beta-catenin with no gene mutations in synovial sarcoma. Virchows Arch 2001; 438: 23–30.
Li CC, Xu B, Hirokawa M, Qian Z, Yoshimoto K, Horiguchi H, et al. Alterations of E-cadherin, α-catenin and β-catenin expression in neuroendocrine tumors of the gastrointestinal tract. Virchows Arch 2002; 440: 145–154.
Mareel M, Bracke M, Van Roy F . Invasion promoter versus invasion suppressor molecules: the paradigm of E-cadherin. Mol Biol Rep 1994; 19: 45–67.
Behrens J, Mareel MM, Van-Roy FM . Dissecting tumor cell invasion: epithelial cells acquire invasive properties after the loss of uvomorulin-mediated cell-cell adhesion. J Cell Biol 1989; 108: 2435–2447.
Liotta LA, Steeg PS, Stetler-Stevenson WG . Cancer metastasis and angiogenesis: an imbalance of positive and negative regulation. Cell 1991; 64: 327–336.
Murphy G, Docherty AJ . The matrix metalloproteinases and their inhibitors. Am J Respir Cell Mol Biol 1992; 7: 120–125.
Slaton JW, Inoue K, Perrotte P, El-Naggar AK, Swanson DA, Fidler IJ, et al. Expression levels of genes that regulate metastasis and angiogenesis correlate with advanced pathological stage of renal cell carcinoma. Am J Pathol 2001; 158: 735–743.
Herbst RS, Yano S, Kuniyasu H, Khuri FR, Bucana CD, Guo F, et al. Differential expression of E-cadherin and type IV collagenase genes predicts outcome in patients with stage I non-small cell lung carcinoma. Clin Cancer Res 2000; 6: 790–797.
Turner HE, Nagy Z, Esiri MM, Harris AL, Wass JA . Role of matrix metalloproteinase 9 in pituitary tumor behavior. J Clin Endocrinol Metab 2000; 85: 2931–2935.
Southgate J, Hutton KA, Thomas DF, Trejdosiewicz LK . Normal human urothelial cells in vitro: proliferation and induction of stratification. Lab Invest 1994; 71: 583–594.
Revillion F, Van de walle B, Hornez L, Lefebvre J . Influence of cAMP on E-cadherin expression and cell surface heparan sulfate proteoglycan synthesis in human breast cancer cells. Anticancer Res 1993; 13: 1625–1629.
Sacchi TB, Bani D, Brandi ML, Falchetti A, Bigazzi M . Relaxin influences growth, differentiation and cell-cell adhesion of human breast-cancer cells in culture. Int J Cancer 1994; 57: 129–134.
Franchi A, Gallo O, Boddi V, Santucci M . Prediction of occult neck metastases in laryngeal carcinoma: role of proliferating cell nuclear antigen, MIB-1, and E-cadherin immunohistochemical determination. Clin Cancer Res 1996; 2: 1801–1808.
Papadavid E, Pignatelli M, Zakynthinos S, Krausz T, Chu AC . Abnormal immunoreactivity of the E-cadherin/catenin (alpha-, beta-, and gamma-) complex in premalignant and malignant non-melanocytic skin tumours. J Pathol 2002; 196: 154–162.
Rubin MA, Mucci NR, Figurski J, Fecko A, Pienta KJ, Day ML . E-cadherin expression in prostate cancer: a broad survey using high-density tissue microarray technology. Hum Pathol 2001; 32: 690–697.
Garcia S, Martini F, De Micco C, Andrac L, Hardwigsen J, Sappa P, et al. Immunoexpression of E-cadherin and beta-catenin correlates to survival of patients with hepatocellular carcinomas. Int J Oncol 1998; 12: 443–447.
Hajra KM, Ji X, Fearon ER . Extinction of E-cadherin expression in breast cancer via a dominant repression pathway acting on proximal promoter elements. Oncogene 1999; 18: 7274–7279.
Yoshiura K, Kanai Y, Ochiai A, Shimoyama Y, Sugimura T, Hirohashi S . Silencing of the E-cadherin invasion-suppressor gene by CpG methylation in human carcinomas. Proc Natl Acad Sci U S A 1995; 92: 7416–7419.
Hirano S, Kimoto N, Shimoyama Y, et al. Identification of a neural alpha-catenin as a key regulator of cadherin function and multi-cellular organization. Cell 1992; 70: 293–301.
Knudsen KA, Wheelock MJ . Plakoglobin, or an 83-kD homologue distinct from beta-catenin, interacts with E-cadherin and N-cadherin. J Cell Biol 1992; 118: 671–679.
Rubinfeld B, Souza B, Albert I, Muller O, Chamberlain SH, Masiarz FR, et al. Association of the APC gene product with beta-catenin. Science 1993; 262: 1731–1734.
Rubinfeld B, Souza B, Albert I, Munemitsu S, Polakis P . The APC protein and E-cadherin form similar but independent complexes with alpha-catenin, beta-catenin, and plakoglobin. J Biol Chem 1995; 270: 5549–5555.
Anastasiadis PZ, Reynolds AB . The p120 catenin family: complex roles in adhesion, signaling and cancer. J Cell Sci 2000; 113: 1319–1334.
Calle-Rodrigue RD, Giannini C, Scheithauer BW, Lloyd RV, Wollan PC, Kovacs KT, et al. Prolactinomas in male and female patients: a comparative clinicopathologic study. Mayo Clin Proc 1998; 73: 1046–1052.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Qian, Z., Li, C., Yamasaki, H. et al. Role of E-Cadherin, α-, β-, and γ-Catenins, and p120 (Cell Adhesion Molecules) in Prolactinoma Behavior. Mod Pathol 15, 1357–1365 (2002). https://doi.org/10.1097/01.MP.0000039572.75188.1A
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1097/01.MP.0000039572.75188.1A
Keywords
This article is cited by
-
Aggressive prolactinomas: how to manage?
Pituitary (2020)
-
Medically induced CSF rhinorrhea following treatment of macroprolactinoma: case series and literature review
Pituitary (2018)
-
The Wnt/β-catenin signaling pathway is involved in the antitumor effect of fulvestrant on rat prolactinoma MMQ cells
Tumor Biology (2014)
-
MGMT expression and pituitary tumours: relationship to tumour biology
Pituitary (2013)
-
Expression and Clinical Significance of Wnt Players and Survivin in Pituitary Tumours
Endocrine Pathology (2012)