Abstract
Both β-catenin and E-cadherin are epithelial cell adhesion molecules. In addition, β-catenin is an important element of the Wnt signal transduction pathway, which has been implicated in embryogenesis and carcinogenesis, including the development of endometrial and ovarian endometrioid carcinomas. We hypothesized that the expression pattern of these two adhesion molecules may depend upon the histological subtype of endometrial carcinomas. Therefore, we compared the immunohistochemical expression of β-catenin and E-cadherin in a set of uterine adenocarcinomas matched for high histologic grade, that is, poorly differentiated (International Federation of Gynecology and Obstetrics [FIGO] Grade III) uterine endometrioid carcinomas and uterine serous carcinomas. Seventeen FIGO Grade III endometrioid adenocarcinomas and 17 serous carcinomas were evaluated histologically and immunohistochemically with commercially available monoclonal antibodies against β-catenin and E-cadherin. Nuclear expression of β-catenin was observed in 8 of 17 (47%) endometrioid adenocarcinomas but in none of the serous carcinomas (P = .003). Moderate or strong E-cadherin expression was identified in 7 of 17 (41%) serous carcinomas as opposed to in only 1 of 17 (6%) endometrioid adenocarcinomas (P = .02). The majority of endometrioid adenocarcinomas showed strong β-catenin expression coupled with weak E-cadherin expression; serous carcinomas did not exhibit a comparable trend. Our results indicate that the expression of β-catenin and E-cadherin in high-grade endometrial cancers is strongly associated with histological subtype. These data provide further support for the distinct molecular profiles of endometrioid adenocarcinoma and serous carcinoma. Notably, differences in cell adhesion molecule expression could account for variations in patterns of tumor dissemination. The immunohistochemical staining pattern may also be useful for diagnostic purposes.
Similar content being viewed by others
INTRODUCTION
Based on clinical evidence, it has been suggested that carcinomas of the endometrium can be subdivided into estrogen-related (Type I) and non–estrogen-related (Type II) tumors (1, 2, 3). Morphologically, these two types are most frequently represented by the endometrioid and serous variants of endometrial carcinoma, respectively. More recently, molecular genetic evidence (e.g., the distribution of PTEN, K-ras, and p53 mutations and microsatellite instability) has accumulated, suggesting that different pathobiologic pathways are involved in the evolution of these two types (4, 5).
E-cadherin is a calcium-dependent transmembranous epithelial adhesion molecule, which is linked to cytoskeletal actin filaments through α- and β- (or alternatively γ-) catenin. β-catenin, in addition to its function in this cell adhesion complex, serves as a key element in the Wnt signal transduction pathway, which has been implicated in embryogenesis and carcinogenesis (reviewed in 6, 7, 8, 9, 10, 11). Cytosolic accumulation of β-catenin leads to its complex formation with transcription factors like Tcf/Lef-1, translocation into the nucleus, and induction of transcription of responsive genes, including c-myc (12), cyclin D1 (13), and c-jun and fra-1 (14; Fig. 1). Alterations in E-cadherin expression have been linked to decreased cell–cell adhesion, metastatic potential, tumor dedifferentiation, and deep myometrial invasion in endometrial and other carcinomas (15, 16). Similarly, mutations in the β-catenin–encoding gene CTNNB1 may lead to defective cell adhesion function (17). Previous studies have reported β-catenin mutations that lead to stabilization and accumulation of the molecule in 10–30% and more of both uterine and ovarian endometrioid adenocarcinomas (18, 19, 20, 21, 22, 23, 24). Alterations in β-catenin have not been reported in serous carcinomas.
To evaluate differences in the expression pattern of E-cadherin and β-catenin between the two types of endometrial carcinoma, we chose to compare similarly aggressive high-grade endometrioid adenocarcinomas with serous carcinomas.
MATERIALS AND METHODS
Seventeen cases of International Federation of Gynecology and Obstetrics (FIGO) Grade III endometrioid adenocarcinoma and 17 cases of serous carcinoma were selected from the archives of the New York Presbyterian Hospital. All cases were retrospectively reviewed by the authors, and the diagnoses were confirmed using accepted criteria (3). For demographic details, the reader is referred to previously published studies performed on the same cases (25, 26). One representative formalin-fixed, paraffin-embedded tissue section from each case was submitted for immunohistochemistry. For antigen retrieval, pressure cooking or microwave pretreatment for 15 minutes was performed. Primary antibodies were obtained from Zymed Laboratories Inc. (South San Francisco, CA; E-cadherin, clone HECD-1; working dilution, 1:600) and Transduction Laboratories (Lexington, KY; β-catenin, clone 14; working dilution, 1:400). For visualization of the antigen, the 3,3′-diaminobenzidine/peroxidase-based ChemMate kit (Ventana, Tucson, AZ) was used according to the manufacturer's instructions. All steps were carried out at room temperature.
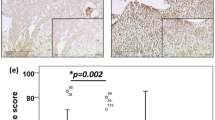
Immunostains were evaluated independently by two authors (PWS and RAS) using a scoring protocol as described elsewhere (27). Briefly, a score ranging from 0 to 12 was determined as the product of staining intensity (on a scale from 0 to 3) and the percentage of positive cells (on a scale from 0 to 4). Scoring categories were defined with scores ranging from 0 (negative), 1 to 3 (weakly), 4 to 7 (moderately), and 8 to 12 (strongly positive). If the two observers’ scores fell into the same category, their evaluation was considered concordant. If their scores fell into different categories, the third author’s (LHE) evaluation was sought, and the disagreement settled by majority vote. For β-catenin, special consideration was given to the subcellular distribution of immunoreactivity (nuclear, membranous, or cytoplasmic).
For statistical analysis, the Student’s t test was used.
RESULTS
Nuclear expression of β-catenin was observed in 8 of 17 endometrioid adenocarcinomas but in none of 17 serous carcinomas (P = .003). Conversely, moderate or strong E-cadherin expression was seen in 7 of 17 serous carcinomas but only in 1 of 17 endometrioid adenocarcinomas (P = .02; Figs. 2 and 3; Table 1). Of the 17 endometrioid adenocarcinomas, 7 showed strong nuclear β-catenin coupled with weak E-cadherin expression, 5 showed strong non-nuclear β-catenin coupled with weak E-cadherin expression, 1 showed strong nuclear β-catenin and strong E-cadherin expression, and 4 showed weak or no β-catenin coupled with weak E-cadherin expression. Thus, there was a tendency of strong β-catenin expression being associated with weak E-cadherin expression.
A comparable trend was not seen in serous carcinomas: of the 17 serous carcinomas, 5 showed strong non-nuclear β-catenin and strong E-cadherin expression, 5 had strong non-nuclear β-catenin and weak E-cadherin expression, 5 had both weak non-nuclear β-catenin and weak E-cadherin expression, and 2 had weak non-nuclear β-catenin with strong E-cadherin expression.
If present in serous carcinomas, β-catenin expression was non-nuclear. E-cadherin expression was restricted to the membranes in both histologic subtypes.
DISCUSSION
We undertook an immunohistochemical analysis of E-cadherin and β-catenin expression in serous carcinomas and poorly differentiated (FIGO Grade III) endometrioid adenocarcinomas. Using this approach, we attempted to minimize a potential bias that might be introduced by comparing relatively indolent low-grade endometrioid adenocarcinomas with the generally highly aggressive serous carcinomas.
Nuclear expression of β-catenin was observed in a subset of endometrioid adenocarcinomas but not in serous carcinomas. Conversely, strong membranous E-cadherin expression was identified predominantly in serous carcinoma as compared with endometrioid adenocarcinoma. Most endometrioid adenocarcinomas showed strong β-catenin expression associated with weak E-cadherin expression. Thus, we found the expression patterns of E-cadherin and β-catenin to be strongly associated with the histologic subtype. This finding provides further support for the separation of endometrioid adenocarcinoma and serous carcinoma as different entities and for their distinct molecular genetic evolution.
Clinical, morphologic and molecular genetic evidence suggests that carcinoma of the endometrium is a heterogeneous group of diseases. Based on clinical data, two major types have been identified: Type I is estrogen related and tends to have a relatively favorable prognosis, whereas Type II is non–estrogen related and follows a more aggressive course (1). Morphologically, these two types can often be correlated with the endometrioid and serous variants of endometrial carcinoma, respectively (2). Recently, molecular genetic findings have supported the view that the two types of endometrial carcinoma emerge via different pathogenetic pathways. Notably, endometrioid adenocarcinomas show mutations in the PTEN tumor suppressor gene in up to 50% and a reported frequency of approximately 28% (5) of microsatellite instability in low-grade tumors, whereas p53 mutations tend to occur late in tumor development. In contrast, serous carcinomas harbor p53 mutations in approximately 90% early on but only rarely show PTEN mutations or microsatellite instability (4, 5).
Mutational analysis of CTNNB1 has revealed stabilizing β-catenin mutations in 10–20% of endometrioid carcinomas of the endometrium and of the ovary (18, 19, 20, 22, 24). Similar figures have been described by Ikeda et al. (23), whereas Mirabelli-Primdahl et al. (21) report a 33–50% incidence of CTNNB1 mutations in endometrial carcinomas; however, the histologic subtype of the analyzed cases was not clearly specified in these two studies. For unknown reasons, the mutations tend to occur in low-grade, low-stage tumors (18, 20, 22, 24) that lack lymph node metastases (28).
Immunohistochemically, nuclear accumulation of β-catenin has been reported in ≤38% of endometrioid tumors (18, 29). This suggests that abnormalities in other elements of the Wnt-signaling pathway may be involved in the pathogenesis of endometrioid carcinomas, the common result being an up-regulation of β-catenin. Saegusa and Okayasu (30) report an association of both β-catenin mutations and nuclear accumulation with squamous differentiation in G1 and G2 endometrioid endometrial and ovarian carcinomas.
The mutational status of CTNNB1 in serous carcinoma is unknown.
Cytoplasmic β-catenin is a key element in the Wnt signal transduction pathway, and its nuclear translocation has been linked to the induction of the c-myc proto-oncogene (12), cyclin D1 (13), and c-jun and fra1 (14), among others. Interestingly, cyclin D1 has been shown to be expressed in 48% of FIGO Grade III uterine endometrioid carcinomas, as opposed to only in 15% of uterine serous carcinomas (26). Thus, the similar percentage of cases positive for both β-catenin and cyclin D1 in high-grade endometrioid adenocarcinomas may reflect the induction of cyclin D1 by β-catenin overexpression.
In nonproliferating cells, the cytosolic β-catenin pool is strictly regulated by a phylogenetically highly conserved mechanism involving a multiprotein complex including the adenomatous polyposis coli tumor suppressor protein (APC), axin, and glycogen synthase kinase 3-β (GSK3β), leading to phosphorylation and subsequent ubiquitin-dependent degradation of β-catenin (reviewed in 6, 7, 8, 9, 10, 11). E-cadherin may contribute to the elimination of β-catenin from the cytoplasm by recruiting it into the adherens junction complex, thereby preventing its translocation into the nucleus (31, 32, 33). On the other hand, as part of the adherens junction complex, β-catenin links E-cadherin through α-catenin to the cytoskeleton (Fig. 1). Thus, malfunction of β-catenin may lead to decreased cell–cell adhesion and facilitate metastatic spread of carcinoma cells (17).
Altered E-cadherin expression has been associated with decreased cell–cell adhesion, metastatic potential, tumor dedifferentiation, and deep myometrial invasion in endometrial and other carcinomas (15, 16). Concerning the endometrioid tumors, our findings coincide with previous observations that poorly differentiated endometrial carcinomas are associated with decreased E-cadherin expression (16). Unfortunately, Sakuragi et al. (16) did not specify the histological subtype of the cases analyzed in their study. As our results show, the distinction of different subtypes of endometrial carcinoma is important, because serous tumors display a divergent expression pattern for E-cadherin.
Because both E-cadherin and β-catenin are involved in cell–cell adhesion, their differential expression pattern may also reflect the different modes of tumor dissemination in endometrioid adenocarcinomas versus serous carcinomas, the latter having a predilection for intraperitoneal dissemination.
Our results suggest that E-cadherin and β-catenin immunostains could be used along with p53 stains (5, 25) to differentiate between these two types of malignancies in diagnostically difficult situations.
In summary, we found the expression patterns of β-catenin and E-cadherin in high-grade endometrial carcinomas to be associated with the histological subtype. These findings support the concept of divergent molecular genetic pathways in different types of endometrial carcinomas. The immunohistochemical staining pattern could be useful for differentiating tumor types for diagnostic purposes.
References
Bokhman JV . Two pathogenetic types of endometrial carcinoma. Gynecol Oncol 1983; 15: 10–17.
Deligdisch L, Cohen CJ . Histologic correlates and virulence implications of endometrial carcinoma associated with adenomatous hyperplasia. Cancer 1985; 56: 1452–1455.
Kurman RJ, Zaino RJ, Norris HJ . Endometrial carcinoma.In: Kurman RJ, editor. Blaustein’s pathology of the female genital tract. 5th ed. New York: Springer Verlag; 2002.
Hedrick L . Endometrial cancer.In: Vogelstein B, Kinzler K, editors. The genetic basis of human cancer. New York: McGraw-Hill; 1998.
Lax SF, Kendall B, Tashiro H, Slebos RJ, Hedrick L . The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer 2000; 88: 814–824.
Morin PJ . β-catenin signaling and cancer. Bioessays 1999; 21: 1021–1030.
Behrens J . Cadherins and catenins: role in signal transduction and tumor progression. Cancer Metastasis Rev 1999; 8: 15–30.
Beavon IRG . The E-cadherin-catenin complex in tumour metastasis: structure, function and regulation. Eur J Cancer 2000; 36: 1607–1620.
Polakis P . Wnt signaling and cancer. Genes Dev 2000; 14: 1837–1851.
Peifer M, Polakis P . Wnt signaling in oncogenesis and embryogenesis—a look outside the nucleus. Science 2000; 287: 1606–1609.
Wijnhoven BPL, Dinjens WNM, Pignatelli M . E-cadherin-catenin cell-cell adhesion complex and human cancer. Br J Surg 2000; 87: 992–1005.
He T-C, Sparks AB, Rago C, Hermeking H, Zawel L, Da Cost LT, et al. Identification of c-MYC as a target of the APC pathway. Science 1998; 281: 1509–1512.
Tetsu O, McCormick F . β-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature 1999; 398: 422–426.
Mann B, Gelos M, Siedow A, Hanski ML, Gratchev A, Ilyas M, et al. Target genes of β-catenin T cell–factor lymphoid-enhancer-factor signaling in human colorectal carcinomas. Proc Natl Acad Sci U S A 1999; 96: 1603–1608.
Fujimoto J, Ichigo S, Hirose R, Sakaguchi H, Tamaya T . Suppression of E-cadherin and alpha- and beta-catenin mRNA expression in the metastatic lesions of gynecological cancers. Eur J Gynaecol Oncol 1997; 18: 484–487.
Sakuragi N, Nishiya M, Ikeda K, Ohkouch T, Furth EE, Hareyama H, et al. Decreased E-cadherin expression in endometrial carcinoma is associated with tumor dedifferentiation and deep myometrial invasion. Gynecol Oncol 1994; 53: 183–189.
Oyama T, Kanai Y, Ochiai A, Akimoto S, Oda T, Yanagihara K, et al. A truncated beta-catenin disrupts the interaction between E-cadherin and alpha-catenin: A cause of loss of intercellular adhesiveness in human cancer cell lines. Cancer Res 1994; 54: 6282–6287.
Fukuchi T, Skamoto M, Tsuda H, Maruyama K, Nozawa S, Hirohashi S . β-catenin mutation in carcinoma of the uterine endometrium. Cancer Res 1998; 58: 3526–3528.
Palacios J, Gamallo C . Mutations in the β-catenin gene (CTNNB1) in endometrioid ovarian carcinomas. Cancer Res 1998; 58: 1344–1347.
Kobayashi K, Sagae S, Nishioka Y, Tokino T, Kudo R . Mutations of the β-catenin gene in endometrial carcinomas. Jpn J Cancer Res 1999; 90: 55–59.
Mirabelli-Primdahl L, Gryfe R, Kim H, Millar A, Luceri C, Dale D, et al. β-catenin mutations are specific for colorectal carcinomas with microsatellite instability but occur in endometrial carcinomas irrespective of mutator pathway. Cancer Res 1999; 59: 3346–3351.
Wright K, Wilson P, Morland S, Campbell I, Walsh M, Hurst T, et al. β-catenin mutation and expression analysis in ovarian cancer: exon 3 mutations and nuclear translocation in 16% of endometrioid tumours. Int J Cancer 1999; 82: 625–629.
Ikeda T, Yoshinaga K, Semba S, Kondo E, Ohmori H, Horii A . Mutational analysis of the CTNNB1 beta-catenin gene in human endometrial cancer: frequent mutations at codon 34 that cause nuclear accumulation. Oncol Rep 2000; 7: 323–326.
Schlosshauer PW, Pirog EC, Levine RL, Hedrick Ellenson L . Mutational analysis of the CTNNB1 and APC genes in uterine endometrioid carcinoma. Mod Pathol 2000; 13: 1066–1071.
Soslow RA, Shen PUF, Chung MH, Isacson C . Distinctive p53 and mdm2 immunohistochemical expression profiles suggest different pathogenetic pathways in poorly differentiated endometrial carcinoma. Int J Gynecol Pathol 1998; 17: 129–134.
Soslow RA, Shen PUF, Chung M, Isacson C, Baergen RN . Cyclin D1 expression in high grade uterine carcinoma correlates with histologic subtype and estrogen receptor expression. Int J Gynecol Pathol 2000; 19: 329–334.
Remmele W, Schicketanz K-H . Immunohistochemical determination of estrogen and progesterone receptor content in human breast cancer. Computer-assisted image analysis (QIC score) vs. subjective grading (IRS). Pathol Res Pract 1993; 189: 862–866.
Saegusa M, Hashimura M, Yoshida T, Okayasu I . β-catenin mutations and aberrant nuclear expression during endometrial tumorigenesis. Br J Cancer 2001; 84: 209–217.
Nei H, Saito T, Yamasaki H, Mizumoto H, Ito E, Kudo R . Nuclear localization of beta-catenin in normal and carcinogenic endometrium. Mol Carcinog 1999; 25: 207–218.
Saegusa M, Okayasu I . Frequent nuclear β-catenin accumulation and associated mutations in endometrioid-type endometrial and ovarian carcinomas with squamous differentiation. J Pathol 2001; 194: 59–67.
Hulsken J, Birchmeier W, Behrens J . E-cadherin and APC compete for the interaction with beta-catenin and the cy-toskeleton. J Cell Biol 1994; 127: 2061–2069.
Fagotto F, Funayama N, Gluck U, Gumbiner BM . Binding to cadherins antagonizes the signaling activity of beta-catenin during axis formation in Xenopus. J Cell Biol 1996; 132: 1105–1114.
Yang S-Z, Kohno N, Yokoyama A, Kondo K, Hamada H, Hiwada K . Decreased E-cadherin augments β-catenin nuclear localization: studies in breast cancer cell lines. Int J Oncol 2001; 18: 541–548.
Acknowledgements
The authors thank Ms. Liang Ying for excellent technical assistance.
Author information
Authors and Affiliations
Corresponding author
Additional information
Peter W. Schlosshauer, M.D., is now at the Department of Pathology at the Mount Sinai School of Medicine, New York, New York.
Rights and permissions
About this article
Cite this article
Schlosshauer, P., Ellenson, L. & Soslow, R. β-Catenin and E-Cadherin Expression Patterns in High-Grade Endometrial Carcinoma Are Associated with Histological Subtype. Mod Pathol 15, 1032–1037 (2002). https://doi.org/10.1097/01.MP.0000028573.34289.04
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1097/01.MP.0000028573.34289.04
Keywords
This article is cited by
-
Risk-stratification machine learning model using demographic factors, gynaecological symptoms and β-catenin for endometrial hyperplasia and carcinoma: a cross-sectional study
BMC Women's Health (2023)
-
SALL-4 and Beta-Catenin Expression in Sinonasal Teratocarcinosarcoma
Head and Neck Pathology (2022)
-
Significance of p53 expression in background endometrium in endometrial carcinoma
Virchows Archiv (2015)
-
Clinical translation for endometrial cancer stem cells hypothesis
Cancer and Metastasis Reviews (2015)
-
Role of epithelial to mesenchymal transition proteins in gynecological cancers: pathological and therapeutic perspectives
Tumor Biology (2014)