Abstract
Some investigators have reported that the histological features of osteosarcoma (OS) arising in elderly patients are different from those in younger patients; however, a molecular biologic study of OS in elderly patients has not been documented. In this study, 23 cases of OS (15 osteoblastic and 8 MFH-like types) and 18 cases of MFH of bone in patients 40 years of age or older were analyzed for mutation of the p53 gene, amplification of the MDM2 gene, and mutation of the H-ras gene, using formalin-fixed paraffin-embedded materials. We also examined the expression of p53, MDM2, and p21WAF1 protein immunohistochemically and assessed the proliferative activities using the monoclonal antibody MIB-1. p53 immunoreactivity was recognized in 5 of 23 OS cases (22%), whereas p53 gene mutations were also detected in 5 of 23 OS cases (22%; osteoblastic [4/15; 27%] and MFH-like [1/8; 18%] types) and in 4 of 18 cases of MFH of bone (22%). There was a statistically significant correlation between p53 immunoreactivity and p53 mutation status in OS (P = .0482). All those cases of osteoblastic OS and MFH of bone that had p53 mutations, with the exception of one case of MFH of bone that had a silent mutation, showed aggressive biologic behavior (dead of disease within 12 mo), in contrast to the MFH-like OS cases (alive without disease at 22 mo). Three cases of OS (13%) and three cases of MFH of bone (17%) showed immunoreactivity for MDM2. As for gene alteration, three cases of OS (13%) and 3 cases of MFH of bone (17%) demonstrated MDM2 amplification. MDM2 amplification showed a significant correlation with the expression of MDM2 protein in OS (P = .0344). p21WAF1 expression was detected in three cases of OS (13%) and in six cases of MFH of bone (33%). MDM2 alteration and p21WAF1 expression were not observed in any of the cases of MFH-like OS. MIB-1-LI showed a statistically significant correlation with p53 immunoreactivity and MDM2 immunoreactivity in OS (P = .0307 and P = .0358, respectively). H-ras mutation at Codons 12 and 13 was not recognized in any of the cases of OS or MFH of bone. In conclusion, although treatment differences during the time of study make it difficult to compare survival analysis, in the current study, p53 mutation in osteoblastic OS and MFH of bone in elderly patients seemed to be closely associated with the progression of the tumor, which was not the case in MFH-like OS. Furthermore, MDM2 alteration and p21WAF1 expression were demonstrated only in osteoblastic OS and MFH of bone, whereas they were not recognized in MFH-like OS. Although the number of patients in this analysis was small, it would appear that MFH-like OS may have some characteristic biologic aspects when compared with osteoblastic OS and MFH of bone in elderly patients.
Similar content being viewed by others
INTRODUCTION
Osteosarcoma (OS) is a primary malignant bone tumor that frequently arises in younger patients, occurring less commonly in adults or the elderly (1, 3). In contrast, malignant fibrous histiocytoma (MFH) of bone is a malignant bone tumor that frequently arises in adults and the elderly. In the case of OS arising in elderly patients, MFH-like type has been noted more frequently than osteoblastic type (2, 3). In addition, Naka et al. (4) reported that patients with MFH-like OS had a favorable survival compared with patients with osteoblastic OS or MFH of bone and that the proliferative activity in MFH-like OS tended to be lower than in osteoblastic OS or MFH of bone, using Ki-67 as the proliferative marker.
p53 gene mutation has been investigated extensively in mesenchymal tumors, and it has been suggested that the alterations of p53 play a potential role in tumorigenesis and tumor progression. In OS, Yokoyama et al. (4, 5) reported that most cases with p53 mutation were associated with recurrence or metastatic lesions, suggesting the possibility that p53 mutation plays an important role in tumor progression. The MDM2 gene codes protein with the ability to bind to p53 and inhibits the transcriptional activity of p53 (6). Gene amplification of MDM2 has been described as the pathway of tumorigenesis or tumor progression in various sarcomas (5, 6, 7). In OS, gene amplification of MDM2 was found to be correlated with recurrence or metastasis in some previous studies (5, 9, 10), but not in others (11).
H-ras gene mutation has been investigated in various soft-tissue sarcomas (12, 13). With regard to OS, K-ras and N-ras gene mutations have been found in previous studies (5, 14), however H-ras gene mutation has not been detected (4, 15).
Although OS arising in elderly patients has been analyzed in several reports clinicopathologically, the immunohistochemical and molecular biological findings have not been analyzed in detail. In the present study, we analyzed the immunohistochemical and molecular abnormalities of p53, MDM2, and H-ras in OS and MFH of bone arising in patients ≥40 years of age.
MATERIALS AND METHODS
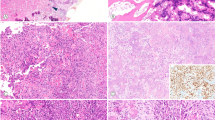
Two hundred and forty-seven patients with osteosarcoma (OS) and 72 patients with malignant fibrous histiocytoma (MFH) of bone were registered in the bone tumor files of the Department of Anatomic Pathology, Graduate School of Medical Sciences, Kyushu University, Japan between 1965 and 1999. Formalin-fixed, paraffin-embedded tissue from a total of 23 cases of OS in patients aged ≥40 years (15 osteoblastic and 8 MFH-like types), and 18 cases of MFH of bone in patients within the same age group were used for immunohistochemical and molecular analyses. The number of evaluated histologic slides ranged from 3 to 12, with a mean of 5 slides per case. We defined osteoblastic OS as a tumor with a prominent lacelike osteoid formation produced by rounded or polygonal atypical cells (Fig. 1A). Almost all the osteoblastic OS cases demonstrated predominantly or entirely osteoblastic lesions. MFH-like OS was defined as a tumor with prominent MFH-like areas that consisted of a mixture of pleomorphic fibroblast-like spindle-shaped and histiocyte-like round cells arranged in a storiform pattern, together with a focal tumor osteoid formation, where the osteoblastic areas never exceeded 50% of the entire lesion (Fig. 1B–C).
A, osteoblastic OS (Case OS8). The lesion is composed of atypical round to polygonal cells with a lace-like osteoid formation (200×). B, MFH-like OS (Case OS17). Microscopically, the majority of the lesion is characterized by MFH-like areas consisting of a mixture of fibroblast-like spindle-shaped cells in a storiform pattern and histiocyte-like round to polygonal cells (150×). C, tumor osteoid formation is evident focally in MFH-like areas (200×). D, MFH of bone (Case MFH11). Histologically, atypical polygonal to short spindle cells can be seen proliferating in a storiform pattern. No tumor osteoid was observed within examined specimens (150×).
Furthermore, we defined MFH of bone as a tumor with a proliferation of atypical polygonal to short spindle cells in a storiform pattern, but with no tumor osteoid formation (Fig. 1D). Diagnoses were based on hematoxylin-eosin (HE) staining, with sampling specimens being decalcified when needed. Immunohistochemical studies using the streptavidin-biotin peroxidase (SAB) method were performed when it was necessary to rule out other lesions. Survival data were available for 21 of the OS cases (14 osteoblastic and 7 MFH-like types) and 17 of the cases of MFH of bone, with a follow-up ranging from 3 to 130 (median, 35.6) months in OS and 2 to 128 (median, 30.7) months in MFH of bone. We assessed the correlation between the clinicopathologic factors (gender, histological subtype, histological grading, and mitotic rate) and the results of both immunohistochemical and molecular analyses.
Immunohistochemistry
Immunohistochemical analysis was performed using mouse IgG monoclonal antibodies against Ki-67 (MIB-1; 1:100, Immunotech, Marseille, France), p53 (PAb1801, 1:100, Oncogene Science, New York, NY), MDM2 (IF2; 1:40, Oncogene Research Products, Cambridge, MA), and p21/WAF1 (EA10; 1:100, Oncogene Research Products). PAb1801 reacts with both mutant and wild-type human p53 protein, and IF2 recognizes an epitope in the amino terminal portion of human mdm-2 protein that corresponds to the p53-binding site.
Four-micron-thick histologic sections were cut, mounted on glass slides coated by 3-aminopropyltriethoxysilane, and air-dried overnight at room temperature. The sections were deparaffinized in xylene and dehydrated in ethanol. After dehydration, the endogenous peroxidase was blocked by methanol containing 3% H2O2 for 30 minutes. For staining with the above antibodies, specimens were pretreated with citrate buffer (0.01 mol/L citric acid: pH, 6.0) four times, each time for 5 min at 100° C in a microwave oven. Sections were incubated with the primary antibody at 4° C overnight, followed by staining with a streptavidin-biotin-peroxidase kit (Nichirei, Tokyo, Japan).
The sections were then finally reacted in a 3,3′ diaminobenzidine, peroxytrichloride substrate solution, counterstained with methyl green or hematoxylin, and then mounted. As for p53, MDM2 and WAF1, when ≥10% of the nuclei in the tumor cells were stained, it was interpreted as a positive result. The MIB-1–labeling index (MIB-1-LI) was determined as the percentage of positive cells by counting the positively stained nuclei in ≥1,000 tumor cells.
Polymerase Chain Reaction–Single-Strand Conformation Polymorphism for p53
Genomic DNA was purified using standard proteinase K digestion and phenol–chloroform extraction methods. The obtained DNA was subjected in a total volume of 5 μL containing 10 mm Tris–HCL (pH 8.3), 50 mm KCl, 2.0 mm MgCl2, 25 mm of dNTP, 0.25 U Taq DNA polymerase, and 0.2 μm of each of the primers. Mutations of the p53 gene were examined from exons 5 to 9. The sequences of the primers are summarized in Table 1. PCR was carried out using a programmable thermal cycler (PTC-100TM: MJ Research Inc., Watertown, MA) for 40 cycles after the first denaturation at 95° C for 1 minute (95° C for 1 min, 66° C for 1 min, and 72° C for 2 min). PCR products were electrophoresed through 2.0% agarose gel with ethidium bromide. The DNA band was purified using a SUPREC tube (TAKARA Biomedicals, Japan) and the products were reamplified for 25 cycles. Five microliters of the reamplified products was diluted 1:1 in loading buffer (94% formamide, 10 mg bromphenol blue, and 0.05% xylene cyanol). Each sample was denatured at 96° C for 5 min and chilled on ice, then a total of 6 μL of the samples was applied onto gel containing 12.5% acrylamide (GenePhor, Amersham Pharmacia Biotech, Uppsala, Sweden). Single-strand conformation polymorphism was performed using a DNA fragment analyzer (GenePhor, Amersham Pharmacia Biotech) at 600 V, 25 mA, 15 W, and 5° C, for 120 minutes. The bands were visualized by a DNA Silver Staining Kit (GenePhor, Amersham Pharmacia Biotech).
Differential PCR for MDM2
The differential PCR method for detecting amplification of MDM2 was based on a modification of a reported method using PAH as the internal control (16). DNA samples were added to a PCR mix with a total volume of 25 μL containing 10 mm Tris-HCl (pH, 8.3), 50 mm KCl, 1.5 mm MgCl2, 0.25 mm dNTP, 0.5 U Taq DNA polymerase, and 0.5 μm of each of the primers. The sequences of the primers are summarized in Table 1. PCR was carried out for 30 cycles after the first denaturation at 95° C for 1 minute (94° C for 1 min, 50° C for 1 min, and 70° C for 1 min). DNA of the SA-1 cell line (American Type Cell Collection, Rockville, MD), which is known to show seven-fold amplification of the MDM2 gene by Southern blot analysis, was used as a positive control. After the amplification, 10 μL of PCR products were electrophoresed through 3.0% agarose gel with ethidium bromide, and the intensities of the DNA products were quantified by National Institutes of Health (NIH) Imaging software, Version 1.56. Comparing the ratio of the intensities of the MDM2 and PAH PCR products for the samples with positive SA-1 cells (seven-fold), the degree of MDM2 amplification was analyzed. Samples showing more than two-fold amplification were judged as showing positive results.
Polymerase Chain Reaction-Restriction Fragment Length Polymorphism (PCR-RFLP) for H-ras
We used the polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) procedure to detect H-ras gene mutations at codons 12 and 13 with strategy primers as reported (17). The sequences of the primers are summarized in Table 1. PCR was carried out using a programmable thermal cycler for 35 cycles (95° C for 1 min, 59° C for 1 min, and 72° C for 2 min). Then, 5 μL of the amplified products that had been diluted 100 times were reamplified by means of nested PCR for 30 cycles (92° C for 15 s, 55° C for 15 s, and 72° C for 30 s). Codon 12 mutations could be detected due to a naturally occurring HpaII site (CCGG) that is lost when the mutation occurs. HpaII digests the 71-bp amplified fragment into two fragments (41 bp and 30 bp), thereby revealing the presence of the normal allele, whereas the mutant allele remains within the undigested 71-bp fragment. With regard to codon 13 mutations, nested primers were used to create a new restriction site for HphI (GGTGA) by changing a T for an A in the second position of codon 14. HphI digests the same 71-bp amplified fragment into two fragments (58-bp and 13-bp), thereby revealing the presence of the normal allele, whereas the mutant allele remains within the undigested 71-bp fragment. The DNA bands were analyzed by 3% agarose gel electrophoresis, stained with ethidium bromide, and then photographed.
DNA Sequencing
Aberrantly migrating bands of p53 were excised from the SSCP gel, and the amplified product was purified by Microcon centrifugal filter devices (Millipore, Bedford, MA). After purification, direct sequencing was carried out by the dideoxy chain termination method using a Perkin Elmer ABI Prism 310 sequence analyzer (Applied Biosystems, Foster City, CA). The primers used for direct sequences were the same sense and anti-sense primers used for the PCR-SSCP in p53.
Statistical Analysis
Fisher's exact test was used to evaluate the association between two dichotomous variables. The difference in the MIB-1-LI between two groups was estimated by Mann–Whitney U test. A P value of <.05 was considered to indicate statistical significance.
RESULTS
Clinical and Histological Findings
Clinicopathologic data for the patients with OS or MFH of bone are summarized in Table 2. There were 11 males and 12 females with OS (M–F ratio, 11:12; osteoblastic [M–F ratio, 3:2] and MFH-like [M–F ratio, 1:3] types), whereas there were 12 males and 6 females with MFH of bone (M–F ratio, 2:1). Although OS arose at various sites, and although the most common site was distal femur (6 cases), followed by proximal tibia (3 cases), this meant that only 9 cases (39%) were found in the bones around the knee. There was no therapeutic history of preoperative chemotherapy or radiotherapy in OS. After the surgery, adjuvant chemotherapy was performed in five OS cases (three osteoblastic and two MFH-like types). Two OS cases had no follow-up and in two more cases the treatment was unknown. In MFH of bone, preoperative radiotherapy was performed in two cases; however, these cases were not considered to be under the influence of radiotherapy histologically.
The histological grade of malignancy was assessed according to the criteria of Dahlin and Unni (18). In OS, 15 cases were found to be Grade 4 (13 osteoblastic and 2 MFH-like types), whereas the other 8 cases were Grade 3 (2 osteoblastic and 6 MFH-like types); however, in MFH of bone, 11 cases were Grade 4, 6 cases were Grade 3, and the other one case was Grade 2. The mitotic rate was classified as high in 9 of the 23 OS cases (9/23; 39%; 8 osteoblastic and one MFH-like types) and in 8 of the 18 MFH of bone cases (7/18; 39%) with ≥15/10 high-power fields.
Immunohistochemistry
Five of the 23 cases of OS (22%; osteoblastic [5/15; 33%] and MFH-like [0/8] types) and 8 of the 18 cases of MFH of bone (44%) demonstrated nuclear accumulation of p53 protein (Fig. 2). There was a significantly positive correlation between p53 immunoreaction and gender in OS (M–F ratio, 5:0, P = .0137; Table 3).
Three of the 23 cases of OS (13%; osteoblastic [3/15; 20%] and MFH-like [0/8] types) and 3 of the 18 cases of MFH of bone (17%) showed immunoreaction for MDM2 (Fig. 3). Coexpression of p53 and MDM2 was observed in two cases in OS (9%), both cases being osteoblastic type (2/15; 13%). No correlation was observed between MDM2 immunoreaction and the other clinicopathologic parameters (Table 3).
Three of the 23 cases of OS (13%; osteoblastic [3/15; 20%] and MFH-like [0/8] types) and 3 of the 18 cases of MFH of bone (33%) demonstrated positive immunoreaction for p21/WAF1. No correlation was observed between p53 immunoreaction or the accumulation of MDM2 and p21 protein (Table 4). p21/WAF1 expression had no statistically significant correlation with p53 mutation in OS or MFH of bone (P = .5392, P > .9999, respectively, Fisher's exact test). Co-assessment of p21/WAF1 and p53 subgroups showed that p21/WAF1+/p53− patients seemed to have a better survival rate compared with other groups; however, the difference was not significant. In two cases of MFH of bone in which preoperative radiotherapy was given, the overexpression of these proteins was not recognized.
p53 Mutation in Exons 5–9
p53 gene mutations were detected in 5 of the 23 cases of OS (21%; osteoblastic [4/15; 27%] and MFH-like [1/8; 13%] types) and in 4 of the 18 cases of MFH of bone (22%) by molecular biological analysis (Fig. 4A–D). As for the type of base substitution, all five cases were missense mutations (four osteoblastic and one MFH-like type) in OS, whereas three cases were missense mutations and one case was a silent mutation in MFH of bone (Table 5). One case in osteoblastic OS showed two mutations at codons 228 and 242. Four mutations were transition (three osteoblastic and one MFH-like types) and one mutation was transversion among five mutations of OS, whereas all four cases were transition in MFH of bone. All patients with p53 mutation in osteoblastic OS and MFH of bone, including one osteoblastic OS case, in which postoperative chemotherapy were given died within 12 months, except for one case with missense mutation of p53 gene in MFH of bone. There were three osteoblastic OS cases with both p53 immunoreaction and concordant p53 mutation, in addition to three cases of MFH of bone (two missense and one silent mutations). There was a statistically correlation between p53 immunoreactivity and p53 mutation status in OS arising in patients ≥40 years of age (P = .0482), but not in MFH of bone in the same group (Table 4). According to the subtype of OS, precise statistical analysis could not be assessed between p53 immunoreaction and p53 mutation or prognosis because of the small number of cases. No positive relationship was detected between p53 mutation and clinicopathologic parameters (Table 3).
PCR-SSCP analysis at Exon 8 (A) and Exon 7 (B). Abnormal shifted bands are evident in Case OS17 (MFH-like OS; A) and in Case OS 3 (osteoblastic OS; B), respectively. Direct DNA sequencing of Exon 8 in Case OS17 (C) and of Exon 7 in Case OS3 (D). (C, Case OS17, Codon 275, TGT to TAT; D, Case OS3, Codons 228 and 242, GAC to GGC and TAC to TGC, respectively).
MDM2 Amplification
Three of the 23 cases of OS (13%; osteoblastic [3/15; 20%] and MFH-like OS [0/8] types) and 3 of the 18 cases of MFH of bone (17%) showed MDM2 amplification (Fig. 5). MDM2 amplification had a significantly positive correlation with MDM2 immunoreactivity only in the OS cases (P = .0344), and not in the MFH of bone cases (Table 6). There was no correlation between p53 mutation and MDM2 amplification in OS or MFH of bone. Two cases with osteoblastic OS showed both p53 mutation and MDM2 amplification. Amplification of the MDM2 gene also showed no correlation with clinicopathologic parameters (Table 3).
H-ras Mutation Status
In this study, H-ras mutation at codons 12 and 13 was not detected in any of the 23 OS cases (0/23; 0%) nor in any of 18 cases of MFH of bone (0/18; 0%).
MIB-1–Labeling Index
MIB-1-LI ranged from 10.1 to 36.8 (mean, 19.6) in OS (osteoblastic type: mean, 21.1; MFH-like type: mean, 16.6), whereas it ranged from 13.9 to 39.6 (mean, 24.6) in MFH of bone. The MIB-1-LI of MFH-like OS was significantly lower than that of MFH of bone (P = .0055, Fisher's exact test). The OS cases with a high MIB-1-LI tended to have a poor prognosis, but the difference was not significant. MIB-1-LI was positively correlated with p53 immunoreactivity and MDM2 immunoreactivity in OS (P = .0307 and P = .0358, respectively; Table 7). In MFH of bone, p53 immunoreactivity was correlated with high MIB-1-LI (P = .0410; Table 7). No correlation was recognized between MIB-1-LI and p53 mutation, MDM2amplification or p21WAF1.
DISCUSSION
OS is the most common primary malignant bone tumor, frequently affecting the region around the knee in younger patients, but occurring less commonly in elderly patients. In fact, it has been reported that only approximately 10% of patients with OS are ≥60 years of age (1). Histological features in elderly OS cases are different from those in younger cases, with MFH-like OS occurring more commonly than osteoblastic type (1, 2). As for the prognosis according to the subtype of OS, Naka et al. reported that MFH-like OS showed a more favorable prognosis than osteoblastic OS with regard to 5-year survival (4). In contrast, some reports stated MFH-like OS was frequently seen in younger patients. Moreover, Dahlin et al. proposed that MFH-like OS falls within the spectrum of conventional OS, but this hypothesis is still controversial (3).
In previous reports of OS, the expression of p53 protein was recognized in 15 to 72% of OS cases (9, 19, 20, 21, 22). Lanardo et al. (9) reported that p53 immunoreactivity was observed to be more likely to occur in elderly patients than in younger patients (27%). On the other hand, Naka et al. (4) revealed that there was no difference regarding the expression of p53 protein between patients of ≥40 years of age (25%) and those of <40 years of age (24%). In addition, with regard to the subtype of OS, they reported that p53 immunoreaction was not observed in MFH-like OS (0/6, 0%); however, it was rather frequently observed in osteoblastic OS (66.7%). These data, including our results, suggest that p53 mutations may play a less important role in MFH-like OS than in osteoblastic OS from an immunohistochemical aspect.
p53 mutations were found in 15 to 31% of OS cases in previous studies (5, 19, 23, 24, 25, 26, 27). Mutations of the p53 gene in patients of <40 years of age have been reported in 13% (4/30; 24) and 16% (6/38; 13), whereas among those of ≥40 years of age they were seen in 20% (1/5) (24) and 14% (1/7) (14). As for the subtype of OS with p53 mutation, osteoblastic type was more common than other subtypes. However, p53 mutations were not detected in MFH-like OS in the previous studies. Taking the current findings into consideration together with previous reports of immunohistochemical and mutation analysis of p53, MFH-like OS dose not seem to be greatly concerned with p53 gene mutation. In fact, in our series, p53 mutation was observed in only one of the 8 MFH-like OS cases, whereas 4 of 15 osteoblastic OS cases demonstrated p53 gene alteration.
The correlation between p53 nuclear accumulation or its mutation and prognosis in sarcoma is controversial. Although Kawai et al.(28) showed that the nuclear immunoreaction of p53 protein was correlated with poor prognosis in various soft-tissue sarcomas, other reports showed no association (19). In the current study, p53 immunoreaction demonstrated no association with prognosis in OS, indicating that it was less correlated with tumor progression in elderly patients. On the other hand, p53 mutation was observed to be more common in recurrent or metastatic lesions, and it has been reported that the mutation of p53 may have some correlation with tumor progression (5). In our study, although the number of patients was small, all those cases of osteoblastic OS and MFH of bone that hadp53 mutations, with the exception of one case of MFH of bone that had a p53 silent mutation, demonstrated progressive behavior (dead of disease [DOD], within 12 months), in contrast to the cases of MFH-like OS (alive without disease [AWD], 22 months). Although substantial improvement in chemotherapy and differences in treatment during the very large time interval covered by this study make it difficult to compare survival analysis between the two subtypes of OS, in MFH-like OS, p53 mutation may be less correlated with the progression of the tumor than it is in osteoblastic OS or MFH of bone.
Gene amplification of MDM2 has been described as the pathway of tumorigenesis or tumor progression in various sarcomas (6, 7, 8). MDM2 amplification in OS has been detected in 0 to 27% of cases (5, 6, 9, 10, 11, 25, 29). Some investigators have reported that MDM2 amplification was recognized more frequently in metastatic or recurrent OS, indicating that MDM2 amplification may be correlated with tumor progression (5, 9, 10). In our study, MDM2 amplification had no relationship with progression either in OS or in MFH of bone. Yokoyama et al.(5) reported that MDM2 amplification and p53 mutation coexisted in 2 of 17 OS cases in progressive tumors. In the current study, the coexistence of p53 mutation and MDM2 amplification was observed in two cases of osteoblastic OS with both patients dying within 6 months (DOD at 4 and 6 mo). Although most studies of MDM2 amplification have been conducted in younger patients, there was a report that one of four OS cases among patients of ≥40 years of age had MDM2 amplification (25%) (10). In the current study, MDM2 amplification was recognized in 3 of 23 cases of OS (13%). As for the subtype of OS, MDM2 amplification was detected in only osteoblastic cases (3/15, 20%), and not in MFH-like OS cases. These results would seem to indicate that MDM2 gene alteration shows different patterns in the different subtypes of OS.
p21 protein product is believed to block cyclin and cyclin-dependent kinase (CDK) complex activity and prevents the passage of cycling cells from G1 to the S phase in a p53-dependent or p53-independent manner (30, 31, 32). In this study, p21WAF1 expression did not show any significant correlation with p53 immunoreactivity or p53 mutation either in OS or in MFH of bone. McClelland et al. (33) demonstrated that a subgroup of p21+/p53− patients had good survival characteristics with regard to breast cancer. In the current study, this subgroup had no association with survival or MIB1-LI either in OS or in MFH of bone. In all MFH-like OS cases, the expression of p21 protein was not detected, and MFH-like OS showed a different pattern when compared with osteoblastic OS or MFH of bone.
Concerning the study of OS, K-ras gene mutation was detected in 2 of 17 cases (5), and N-ras gene mutation was seen in one of 28 cases (14). In contrast, H-ras gene mutation has not been found in OS (15). In our series, H-ras gene mutation was not detected in any of the cases of OS. Bohle et al. (12) reported that H-ras gene mutation was detected in 9 of 32 soft tissue MFH cases (28%), whereas Yoo et al. (13) described it in 6 of 27 soft tissue MFH cases (22%). In the current study, MFH of bone had no H-ras gene mutation. These results suggest that MFH of bone may have some different molecular aspects from MFH of soft tissue.
The MIB-1 monoclonal antibody has been used prominently as an indicator of proliferating activity in malignant tumors, and as a prognostic factor in some malignant tumors (34, 35, 36). Lanardo et al. (9) revealed that a significantly positive correlation was not found between p53 immunoreactivity and the proliferative rate as assessed by MIB-1-LI in OS. In the current study, MIB-1-LI had a significant relationship with p53 and MDM2 immunoreactivity in all the histologic subtypes of OS. p53 and MDM2 immunoreactivity may be considered as useful factors of proliferative activity in OS arising in elderly patients.
In conclusion, p53 mutation was recognized in osteoblastic OS, MFH-like OS, and MFH of bone in elderly patients. Although the number of patients in this study was small, those cases of osteoblastic OS and MFH of bone that had p53 mutations had progressive tumors, in contrast to the MFH-like OS cases. In addition, the pattern of MDM2 alteration and p21WAF1 expression in MFH-like OS was different from that in osteoblastic OS and MFH of bone. It would thus seem likely that MFH-like OS has some characteristic biologic aspects when compared with osteoblastic OS and MFH of bone in elderly patients, however, a further detailed analysis of a large number of cases, including cases in younger patients is necessary.
References
Huvos AG . Osteogenic sarcoma of bones and soft tissues in older persons: a clinicopathologic analysis of 117 patients older than 60 years. Cancer 1986; 57: 1442–1449.
Brooks S, Starkie CM, Clarke NMP . Osteosarcoma after the fourth decade: a clinico-pathological review. Arch Orthop Trauma Surg 1985; 104: 100–105.
Dahlin DC, Unni KK . Osteosarcoma of bone and its important recognizable varieties. Am J Surg Pathol 1977; 1: 61–72.
Naka T, Fukuda T, Shinohara N, Iwamoto Y, Sugioka Y, Tsuneyoshi M . Osteosarcoma versus malignant fibrous histiocytoma of bone in patients older than 40 years: a clinicopathologic and immunohistochemical analysis with special reference to malignant fibrous histiocytoma-like osteosarcoma. Cancer 1995; 76: 972–984.
Yokoyama R, Schneider-Stock R, Radig K, Wex T, Roessner A . Clinicopathologic implications of MDM2, p53 and K-ras gene alterations in osteosarcomas: MDM2 amplification and p53 mutations found in progressive tumors. Pathol Res Pract 1998; 194: 615–621.
Oliner JD, Pietenpol JA, Thiagalingam S, Gyuris J, Kinzler KW, Vogelstein B . Oncoprotein MDM2 conceals the activation domain of tumor suppressor p53. Nature 1993; 362: 857–860.
Habuchi T, Kinoshita H, Yamada H, Kakehi Y, Ogawa O, Wu WJ, et al. Oncogene amplification of urothelial cancers with p53 gene mutation or MDM2 amplification. J Natl Cancer Inst 1994; 86: 1331–1335.
Lianes P, Orlow I, Zhang ZF, Oliva MR, Sarkis AS, Reuter VE, et al. Altered patterns of MDM2 and p53 expression in human bladder cancer. J Natl Cancer Inst 1994; 86: 1325–1330.
Lanardo F, Ueda T, Huvos AG, Healey J, Ladanyi M . p53 and MDM2 alterations in osteosarcomas: correlation with clinicopathologic features and proliferative rate. Cancer 1997; 79: 1541–1547.
Ladanyi M, Cha C, Lewis R, Jhanwar SC, Huvus AG, Healey JH . MDM2 gene amplification in metastatic osteosarcoma. Cancer Res 1993; 53: 16–18.
Nakayama T, Toguchida J, Wadayama B, Kanoe H, Kotoura Y, Sasaki MS . MDM2 gene amplification in bone and soft tissue tumors: associated with tumor progression in differentiated adipose tissue tumors. Int J Cancer 1996; 64: 342–346.
Bohle RM, Brettreich S, Repp R, Borkhardt A, Kosmehl H, Altmannsberger HM . Single somatic ras gene point mutation in soft tissue malignant fibrous histiocytomas. Am J Pathol 1996; 148: 731–738.
Yoo J, Robinson RA, Lee JY . H-ras and K-ras gene mutations in primary human soft tissue sarcoma: concomitant mutations of the ras gene. Mod Pathol 1999; 12: 775–780.
Pompetti F, Rizzo P, Simon RM, Freidlin B, Mew DJ, Pass HI, et al. Oncogene alterations in primary, recurrent, and metastatic human bone tumors. J Cell Biochem 1996; 63: 37–50.
Antillón-Klüssmann F, García-Delgado M, Villa-Elízaga I, Sierrasesumaga L . Mutational activation of ras genes is absent in pediatric osteosarcoma. Cancer Genet Cytogenet 1995; 79: 49–53.
Reid AH, Tsai MM, Venzon DJ, Wright CF, Lack EE, O'Leary TJ . MDM2 amplification, p53 mutation, and accumulation of the p53 gene product in malignant fibrous histiocytoma. Diagn Mol Pathol 1996; 5: 65–73.
Capella G, Matias-Guiu X, Ampudia X, Leiva A, Perucho M, Prat J . Ras oncogene mutations in thyroid tumors. Diagn Mol Pathol 1996; 5: 45–52.
Unni KK, Dahlin DC . Grading of bone tumors. Semin Diagn Pathol 1984; 1: 165–172.
Mousses S, McAuley L, Bell RS, Kandel R, Andrulis IL . Molecular and immunohistochemical identification of p53 alterations in bone and soft tissue sarcomas. Mod Pathol 1996; 9: 1–6.
Bodey B, Gröger AM, Bodey B Jr, Siegel S, Kaiser HE . Immunohistochemical detection of p53 protein overexpression in primary human osteosarcomas. Anticancer Res 1997; 17: 493–498.
Wadayama B, Toguchida J, Yamaguchi T, Sasaki MS, Kotoura Y, Yamamuro T . p53 expression and its relationship to DNA alterations in bone and soft tissue sarcomas. Br J Cancer 1993; 68: 1134–1139.
Ueda Y, Dockhorn-Dworniczak B, Blasius S, Mellin W, Wuisman P, Bocker W, et al. Analysis of mutant p53 protein in osteosarcomas and other malignant and benign lesions of bone. J Cancer Res Clin Oncol 1993; 119: 172–178.
Radig K, Schneider-Stock R, Haeckel C, Neumann W, Roessner A . p53 gene mutation in osteosarcomas of low-grade malignancy. Hum Pathol 1998; 29: 1310–1316.
Radig K, Schneider-Stock R, Oda Y, Neumann W, Mittler U, Roessner A . Mutation spectrum of the p53 gene in highly malignant human osteosarcomas. Gen Diagn Pathol 1996; 142: 25–32.
Miller CW, Aslo A, Won A, Tan M, Lampkin B, Koeffler HP . Alterations of the p53, Rb and MDM2 genes in osteosarcoma. J Cancer Res Clin Oncol 1996; 122: 559–565.
Smith-Sorensen B, Gebhardt MC, Kloen P . Screening for TP53 mutations in osteosarcomas using constant denaturant gel electrophoresis (CDGE). Hum Mutat 1993; 2: 274–285.
Toguchida J, Yamaguchi T, Ritchie B, Beauchamp RL, Dayton SH, Herrera GE, et al. Mutation spectrum of the p53 gene in bone and soft tissue sarcomas. Cancer Res 1992; 52: 6194–6199.
Kawai A, Noguchi M, Beppu Y, Yokoyama R, Mukai K, Hirohashi S, et al. Nuclear immunoreaction of p53 protein in soft tissue sarcomas: a possible prognostic factor. Cancer Res 1994; 73: 2499–2505.
Florenes VA, Mælandsmo GM, Forus A, Andreassen A, Myklebost O, Fodstad O . MDM2 gene amplification and transcript levels in human sarcomas: relationship to TP53 gene status. J Natl Cancer Inst 1994; 86: 1297–1302.
El-Deiry WS, Tokino T, Velculescu VE, Levy DB, Parsons R, Trent JM, et al. WAF1, a potential mediator of p53 tumor suppression. Cell 1993; 75: 817–825.
Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ . The p21 cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993; 75: 805–816.
Parker SB, Eichele G, Zhang P, Rawls A, Sands AT, Bradley A, et al. p53-independent expression of p21 Cip1 in muscle and other terminally differentiating cells. Science 1995; 267: 1024–1027.
McClelland RA, Gee JMW, O'Sullivan L, Barnes DM, Robertson JFR, Ellis IO, et al. p21 WAF1 expression and endocrine response in breast cancer. J Pathol 1999; 188: 126–132.
Drobnjak M, Latres E, Pollack D, Karpeh M, Dudas M, Woodruff JM, et al. Prognostic implications of p53 nuclear overexpression and high proliferative index of Ki-67 in adult soft tissue sarcoma. J Natl Cancer Inst 1994; 86: 549–554.
McGuire WL, Clark GM . Prognostic factors and treatment decisions in axillary-node negative breast cancer. N Engl J Med 1992; 326: 1756–1761.
Zehr RJ, Bauer TW, Marks KE, Weltevreden A . Ki-67 and grading of malignant fibrous histiocytomas. Cancer 1990; 66: 1984–1990.
Acknowledgements
We are grateful to Miss Y. Nouzuka for her excellent technical assistance. The English used in this manuscript was revised by Miss K. Miller (Royal English Language Centre, Fukuoka, Japan).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kawaguchi, Ki., Oda, Y., Sakamoto, A. et al. Molecular Analysis of p53, MDM2, and H-ras Genes in Osteosarcoma and Malignant Fibrous Histiocytoma of Bone in Patients Older than 40 Years. Mod Pathol 15, 878–888 (2002). https://doi.org/10.1097/01.MP.0000024264.48690.EA
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1097/01.MP.0000024264.48690.EA
Keywords
This article is cited by
-
Genetically engineered mouse models and human osteosarcoma
Clinical Sarcoma Research (2012)
-
MDM2 and CDK4 immunohistochemistry is a valuable tool in the differential diagnosis of low-grade osteosarcomas and other primary fibro-osseous lesions of the bone
Modern Pathology (2011)
-
RB1 and TP53 pathways in radiation-induced sarcomas
Oncogene (2007)
-
Regulating the p53 pathway: in vitro hypotheses, in vivo veritas
Nature Reviews Cancer (2006)
-
Altered expression and molecular abnormalities of cell-cycle-regulatory proteins in rhabdomyosarcoma
Modern Pathology (2004)