Abstract
Angiosarcomas are rare malignant vascular tumors with a high rate of metastasis involving lungs (most commonly), liver, regional lymph nodes, bone, and other sites. In this study, we have reviewed the clinical presentation and histopathology of 21 cases of extracutaneous angiosarcoma metastatic to the lungs. Tumors with exclusively pleural involvement were excluded. Patients presented with dyspnea, chest pain, and/or hemoptysis lasting a few weeks to months. Radiologically, the most common finding comprised multiple peripheral lung nodules (57%), often accompanied by infiltrates. For 11 cases (52%), the primary tumor was not identified at the time of presentation. Vasoformative areas were identified in 15 cases (71%). Nine cases comprised spindle cells (43%), two contained epithelioid cells (9.5%), and 10 consisted of both spindle and epithelioid cells (48%). Nuclear pleomorphism was at least moderate in all cases. However, five tumors contained regions of minimal nuclear atypia. Hemorrhage, siderophages, and fibrosis were commonly present. Immunohistochemical staining (IHS) was performed on 14 cases. Thirteen tumors showed reactivity for vascular markers. Tumor cells reacted for Von Willebrand factor in 13 of 14 cases, and CD31 and CD34 were each positive in 2/2 cases. Two cases (of nine examined) also expressed cytokeratins. Because the tumor often first presented in the lungs before the primary sarcoma was identified, the clinical impression included both benign and malignant entities. For patients with primary cardiac tumors, symptoms referable to the primary tumor complicated the clinical presentation, and radiologic evaluation supported a clinical diagnostic impression of non-neoplastic pericarditis. Thus, angiosarcoma in the lung may elude diagnosis until histopathologic evaluation of the lung biopsy.
Similar content being viewed by others
INTRODUCTION
This study of noncutaneous angiosarcomas metastatic to the lungs was performed to determine the most common site of origin (where this knowledge was available), the range of clinical presentations, and the histologic differential diagnosis that would occur based on the appearance of the pulmonary lesions. Primary angiosarcomas exhibit a variety of histologic appearances that may mimic carcinoma, other sarcomas, melanoma, and even benign reactive processes (1). Metastatic lesions in the lungs may assume growth patterns that might provoke strong consideration of other diagnoses (2). Because the tumor is relatively rare, only a few series of angiosarcomas metastatic to the lungs have been published, the largest comprising 15 patients treated at the Mayo Clinic from 1950 to 1990 (3). For this study, cases were collected from consultation practice files. Noncutaneous angiosarcomas were specifically selected to further define patient demographics and primary site of metastatic angiosarcoma not derived from the skin.
MATERIALS AND METHODS
The consultative files of the authors were searched for cases of angiosarcoma in the lungs seen between 1989 and 1997. Cases with a known primary tumor in the skin or that comprised entirely pleural-based, metastatic lesions were excluded. Twenty-one cases had hematoxylin and eosin–stained tissue sections available for review. The specimens comprised 18 open lung biopsies, two lobectomies, and one combination open lung biopsy and autopsy sampling of involved lung. Two to 55 slides were examined by the authors (average of 3.6 slides per case). In 10 more recent cases, representative sections of tumor were evaluated by means of immunohistochemical staining (IHS). Formalin-fixed, paraffin-embedded tissue sections were deparaffinized in xylene, subjected to a series of graded alcohols for rehydration, and then placed on a DAKO Autostainer automated stainer (DAKO, Carpenteria, CA) using the following antibodies: von Willebrand factor (DAKO), CD31 (Qbend10, DAKO), CD34 (DAKO), AE1/AE3 cytokeratins (DAKO), and S-100 protein (DAKO). Tissues were pretreated with steam heating in citrate buffer for enhanced antigenicity. Four cases received in consultation were accompanied by immunohistochemical stains performed at the outside institution. These IHS slides were reviewed, and positive internal controls were present to assess for adequate reactivity of vascular and epithelial antibodies.
RESULTS
The patient demographics, presenting signs and symptoms, and treatment are summarized in Table 1. All patients were adults, comprising 17 men and three women (sex unknown in one case). Age ranged from 27 to 71 (n = 20), with a median age of 47.8 years. Follow-up information was obtained for 16 patients: 15 patients succumbed to their disease with an average time of survival of 39 weeks after lung biopsy (range 2–206 weeks). The surviving patient had widespread metastases 38 months after presentation. Survival for the 10 patients with a known primary (three patients lost to follow-up) ranged from two weeks to 206 weeks, with an average of 46 weeks. Survival for the 11 patients with no known primary (three patients lost to follow-up) ranged from four weeks to 64 weeks, with an average of 19 weeks. Two-year crude survival percentages for those with known primary was 28% (seven cases) and for those with no known primary, it was 0% (eight cases).
Presenting symptoms were obtained for 13 patients. Of these, nine presented with hemoptysis with or without dyspnea, fever, and/or chest pain. After hemoptysis, dyspnea was the most common patient symptom, (7/9) followed by chest pain and fatigue (2/9 each). One 33-year-old man with diffuse bilateral infiltrates also presented with fever. Radiologic findings most commonly consisted of nodules or nodular infiltrates (12/18), which were usually bilateral. The second most common pulmonary pattern comprised bilateral infiltrates without nodularity (5/18 cases). All four patients with a known pericardial angiosarcoma also exhibited a pericardial effusion radiologically (4/4). No pericardial effusion was identified in the four patients with a known cardiac parenchymal angiosarcoma (0/4).
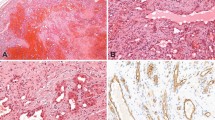
The primary histologic features and immunohistochemical staining results of the cases are presented in Table 2. In all cases, tumor foci were peripheral, multiple, and generally formed nodules. Peripheral lung septae or the subpleural interstitium were involved in all cases (Fig. 1). Tumor extended into surrounding pulmonary alveoli to varying degrees. Lymphangitic spread, when present, usually, but not exclusively, involved septal lymphatics and vessels (Fig. 4A). Circumferential growth around small vessels and bronchioles was noted in seven cases (32%; Fig. 4B). This growth pattern often resulted in tumor thrombi or bronchiole obstruction when tumor penetrated through the walls of the structure. Vasoformative regions were identified in 15 cases (71%). These regions consisted of anastomosing channels lined by mildly to markedly atypical tumor cells. These channels often contained red blood cells (Figs. 3, A–B, and 5A). Solid growth occurred in 17 cases (77%; Fig. 2). Fresh hemorrhage, clusters of siderophages, and extracellular hemosiderin deposition occurred both within tumor nodules and in immediately adjacent lung parenchyma (Fig. 5B).
Ten of the patients had a known primary tumor at the time of lung presentation (10/21, 48%); of these, eight of the primary angiosarcomas were cardiac/pericardial, one was retroperitoneal, and one was mediastinal in location. Eleven patients had no known primary angiosarcoma at the time of presentation (11/21, 52%). Initial clinical diagnostic impressions included pericarditis, vasculitis due to a collagen vascular disease, interstitial pneumonia, sarcoidosis, and metastatic adenocarcinoma (n = 13). The initial pathologic interpretation included the following benign entities: bronchiectasis with healed pneumonia (three cases), idiopathic pulmonary hemosiderosis (two cases), and eosinophilic granuloma (one case). Thus, 6 of the 19 cases (32%) for which an initial pathologic interpretation was obtained consisted of benign entities. Malignant entities favored on the initial pathologic interpretation included angiosarcoma (six cases), high-grade adenocarcinoma (three cases), Kaposi's sarcoma (two cases), malignant fibrous histiocytoma (one case), and epithelioid hemangioendothelioma (one case). Six of the 19 cases (32%) were initially interpreted on microscopic review as angiosarcoma.
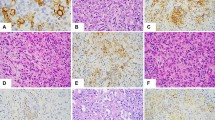
Cytologically, the tumor cells were either spindle or round to polygonal (epithelioid) in shape (Fig. 6, A–B). Ten cases contained a mix of spindled and epithelioid cells. Nine cases were exclusively composed of spindle cells, and two cases were composed of epithelioid cells only. The nuclear to cytoplasmic ratio ranged from generally low in spindle cells to progressively higher for high-grade epithelioid cells. The cytoplasm was lightly eosinophilic, and intracytoplasmic lumina consistent with incipient vessel development was rare (Fig. 6A). Although five cases disclosed areas of minimal nuclear atypia consisting of small, oval nuclei with mild nuclear hyperchromaticity, all cases had at least one focus of higher grade tumor comprising cells with moderate to severe nuclear pleomorphism (including multilobulation, marked hyperchromaticity, coarse chromatin, and irregular nuclear borders with sharp-angled notching). High grade epithelioid cells commonly contained large, vesicular nuclei with macronucleoli (Fig. 6B). Multinucleated tumor giant cells were identified in several cases. Mitotic figures were typically easy to find, and high-grade foci contained cells with atypical division figures.
Immunohistochemically, of 14 cases evaluated, 13 exhibited reactivity for at least one vascular marker. Von Willebrand factor (Factor VIII–related antigen) was positive in 13 of 14 cases where it was used (93%), as were CD31 and CD34 in two of two cases. These cases comprise archival specimens; therefore, CD31 and CD34, recently recognized as useful identifiers of vascular differentiation, were not employed in most of the specimens. Two epithelioid tumors showed strong cytoplasmic staining for AE1/AE3 cytokeratins. However, the same cells also reacted with antibodies to von Willebrand factor.
DISCUSSION
Discovering metastatic angiosarcoma in a lung biopsy is often the initial manifestation of the disease and an unexpected finding in an appreciable percentage of patients with occult extracutaneous angiosarcomas. Metastatic angiosarcoma is usually not a clinical consideration at the time of presentation of these patients. The clinical impression includes benign entities, especially thromboembolic disease and alveolar hemorrhage syndromes, or malignancies such as metastatic adenocarcinoma. In the present study, the initial clinical impression also included pericarditis, vasculitis as a complication of a collagen vascular disease, interstitial pneumonia, sarcoidosis, and metastatic adenocarcinoma (n = 13). Although it is a rare tumor, extracutaneous angiosarcoma most commonly metastasizes to the lungs, and the metastasis rate is at least 50% (number of primary tumors, 266; number of cases with metastases, 136 [51.1%]; 1, 4, 5, 6). In this study, the cases in which a primary tumor site was discovered consisted of the pericardium (four cases), heart (four cases, right atrium location), and one each in the retroperitoneum and mediastinum.
Although this study consists of cases culled from a consultation practice, thereby possibly increasing the number of lesions for which there was no known primary at the time of presentation, other series have found a similar high rate of initial diagnosis occurring with the lung biopsy (3, 7).
Presenting symptoms in this patient group are similar to those reported in other series: hemoptysis, dyspnea, chest pain, or cough, although in one study, weight loss was noted in 40% (6/15 cases; 3, 7, 8). In some instances, tumor metastases have caused hemopneumothorax or diffuse alveolar hemorrage (8). In the present study, the pulmonary symptoms culminated in the patients undergoing lung biopsy. In 12 cases, interpretation of the biopsy findings was made more challenging because of the lack of a known diagnosis of primary angiosarcoma.
Radiologically, pulmonary metastases of angiosarcoma most often comprise bilateral, peripheral nodules, a typical distribution pattern for metastatic tumors to the lungs (2). However, other findings may include a miliary pattern of spread, diffuse alveolar filling, diffuse interstitial infiltrates with a ground glass distribution, pneumothorax, pleural effusions, and solitary endobronchial mass (2, 7, 9). In many instances, there is a combination of bilateral nodules and another finding such as infiltrates which was the finding in our series (3). The radiologic differential diagnosis may consist of infection, metastatic tumor of unknown primary site, multiple pulmonary emboli, Wegener's granulomatosis, Goodpasture's syndrome, and idiopathic pulmonary hemorrhage.
In the current series, the most common known primary site was the heart (including the pericardium). Other series of angiosarcomas metastatic to the lungs concur that the heart and pericardium are the most common sites of origin, once cutaneous angiosarcoma is excluded (7, 9). Angiosarcoma is one of the more common primary malignant tumors of the heart comprising up to one third of the cases (10). Nearly all cardiac angiosarcomas arise in the right atrium or interatrial septum (10, 11). Patients are generally younger compared with individuals with angiosarcomas originating elsewhere; a study of 12 cardiac angiosarcomas found a mean age of 38 years contrasted to a mean age in the 60s for soft tissue angiosarcomas and of 63 years for angiosarcomas arising in the head and neck (n = 32; 1, 4, 6).
Presenting symptoms of cardiac/pericardial angiosarcomas without lung involvement can be virtually identical to those of patients with angiosarcoma metastatic to the lungs. These symptoms include dyspnea, hemoptysis, chest pain, and malaise (10, 13, 14). However, more localizing symptoms may also occur such as a friction rub, diminished heart sounds, tamponade (if a pericardial effusion is present) and angina, arrhythmias, and heart block (if the tumor infiltrates the myocardium; 15). Unfortunately, cardiac/pericardial angiosarcomas are often not diagnosed until late in the disease course (14). Reasons for delayed diagnosis of primary cardiac angiosarcoma encompass the rarity of the tumor compared with benign causes of pericarditis and pericardial effusions, the relative young age of the patients and thus low clinical suspicion of malignancy, the presence of a pericardial effusion that is often bloody but may be negative for malignant cells by cytology, and the sometimes lack of specificity of the symptoms (14, 15). In this series, four patients with pericardial effusions were at first thought to have nonneoplastic disease, the workups of whom included two negative bloody pericardial fluid cytology exams, and two patients were initially treated with prednisone for presumed pericarditis.
By the time that open lung biopsy is performed and reveals metastatic angiosarcoma, the patients have high-stage disease, and the prognosis is grave. In their series of 15 patients treated at the Mayo Clinic from 1950–1990, Patel and Ryu (3) recorded an average survival time of nine months after diagnosis of metastatic angiosarcoma. Adverse prognostic factors portending a more aggressive course in angiosarcomas from all sites include larger size (five cm in one study), older patient age (seventh vs. fourth decade or younger), mitotic rate greater than or equal to 10 division figures per 10 high-power microscopic fields, Ki-67 reactivity of at least 10% in tumor cells, and high histologic grade (1, 6, 16, 17). DNA diploidy does not predict a benign course (5).
There are no histologic features of pulmonary metastases of angiosarcoma that provide clues to the site of origin. The majority of the metastases in this series of patients exhibited a mixed pattern of growth such as solid and vasoformative, a phenomenon also observed in primary angiosarcomas arising from diverse sites (1, 6, 7, 8, 9, 18). The cytologic findings of pure or mixed spindle and epithelioid cells are no different from those of the cellular populations that compose primary angiosarcomas arising throughout the body.
Immunohistochemical antibody markers of endothelial differentiation can elucidate the nature of a vasoformative tumor and distinguish it from its histologic mimics. Antibodies used to detect endothelial differentiation are directed against von Willebrand factor (Factor VIII–related antigen), CD31 glycoprotein, (platelet endothelium cell adhesion molecule, PECAM-1) and CD 34 protein (human hematopoietic progenitor cell antigen; 19, 20). Ulex europeaus lectin stains endothelial cells but also a variety of carcinomas, thus hampering its usefulness in distinguishing between vascular and epithelial neoplasms (21). Although CD31 antibody labels a variety of hematopoietic cells (platelets, megakaryocytes, plasma cells), it rarely stains epithelial tumors and mesotheliomas and then only in a cytoplasmic rather than the more specific membranous pattern (19). CD34 antibody also decorates a number of cell types ranging from fibroblasts to smooth muscle cells, meningothelium, and adipocytes (19).
Comparisons of the sensitivity and specificity of these IHS markers in staining angiosarcomas reveal that, in general, anti–von Willebrand factor and anti-CD31 are the most specific antibodies in detecting a vasoformative phenotype (19, 20, 21, 22). In less differentiated areas, antibody to CD31 is more sensitive than anti–von Willebrand factor (20, 22). CD34 antibody is less reliable in decorating cells that line lymphatic channels and therefore may not be reactive in malignant sarcomas with lymphatic lining cell differentiation (23). A recent review of these markers comparing them to thrombomodulin in 50 vascular and lymphatic tumors and malformations (including eight angiosarcomas) found that thrombomodulin antibody more consistently stained lymphatics and was more sensitive than CD31 in staining angiosarcomas (24).
Angiosarcomas presenting in the lungs may be misinterpreted as a variety of other tumors or as benign processes. In the latter circumstance, this may be compounded by the fact that alveolar hemorrhage or pulmonary emboli is the leading clinical diagnostic consideration. There are four groups of lesions to include in the differential diagnosis: 1) thromboembolic disease, 2) alveolar hemorrhage syndromes, 3) benign vasoformative lesions (pulmonary capillary hemangiomatosis and diffuse pulmonary lymphangiomatosis), and 4) malignancies associated with intravascular or perivascular growth or that produce pseudovascular spaces (see Table 3).
In this study, the initial pathologic interpretations included infarct multiple theromboemboli, idiopathic pulmonary hemosiderosis, bronchiectasis with healed pneumonia, eosinophilic granuloma, and malignancies including high-grade adenocarcinoma, malignant fibrous histiocytoma, epithelioid hemangioendothelioma, Kaposi's sarcoma, sarcoma not otherwise specified, angiosarcoma intravascular lymphoma, and melanoma.
Benign entities were considered because the metastatic foci disclosed one or more of the following features at low to intermediate power: a wedge-shaped, subpleural mass that was fibrotic and/or hemorrhagic, an intra-alveolar proliferation of spindle cells with fibrosis and inflammation, intra-alveolar acute hemorrhage with siderophages, and intravascular deposits of spindle cells and fibrin. At high power, however, neovascularization formed by cytologically atypical cells, solid growth, intravascular deposits containing atypical cells, or invasive growth by atypical cells could be appreciated. The degree of cytologic atypia and/or invasive growth thus precluded diagnosis of a benign entity. In particular, the presence of malignant vessel formation and the marked degree of cytologic atypia excluded Kaposi's sarcoma and epithelioid hemangioendothelioma, whereas immunohistochemical staining results confirming the endothelial nature of the malignant cells excluded the other malignancies in the differential.
Organizing hemorrhage and hemorrhagic inflammatory conditions in the lungs show more air space organization and less nuclear pleomorphism and are associated with extensive fibrosis and inflammation in the surrounding lung parenchyma, a feature unusual in metastatic angiosarcoma. Numerous siderophages may be seen with both benign hemorrhage and metastatic angiosarcoma (7, 9). Rarely, diffuse alveolar hemorrhage (DAH) may occur secondary to the angiosarcoma metastases (8, 18). It is therefore prudent to carefully search for evidence of tumor in a case that otherwise, at low power, exhibits characteristics of DAH.
Diffuse pulmonary lymphangitic spread of carcinomas is a pattern of metastasis that most commonly occurs with stomach, breast, and prostate carcinomas; melanoma; and angiosarcoma (2, 7). The presence of vasoformative areas and reactivity for one and often multiple endothelial markers distinguishes angiosarcoma from the other tumors with lymphangitic spread. Rarely, a carcinoma may diffusely embolize to small pulmonary arteries and arterioles, a condition known as carcinomatous arteriopathy or pulmonary tumor thrombotic microangiopathy. Patients may present with dyspnea and other signs of chronic pulmonary hypertension. This pattern of metastasis is rare and is usually diagnosed at autopsy. Carcinomas most commonly associated with this arteriopathy are those from the stomach, pancreas, breast, liver, and placenta. Histologically, there is widespread thrombus formation comprising a fibrocellular intimal proliferation; multiple tissue sections may be required to find the malignant epithelial cells. Sarcomatoid carcinomas of the lung may histologically contain areas that appear to form vascular channels (26). In the several cases reported, however, each pseudovascular carcinoma also contained foci of identifiable squamous cell carcinoma (26). Immunohistochemical staining in pseudovascular areas reveals epithelial marker reactivity but no staining for endothelial antigens in the tumor cells. It is conceivable that a sarcomatoid carcinoma with nearly complete malignant mesenchymal metaplasia could arise in the lung and be difficult to distinguish from metastatic angiosarcoma; such tumors with endothelial differentiation have been tentatively described in the thyroid gland but not in the lungs (21, 26). Undifferentiated carcinoma is usually more architecturally monotonous than metastatic angiosarcoma and by IHS will show reactivity for epithelial markers rather than endothelial antigens.
Melanoma may resemble epithelioid angiosarcoma and often contains a mixture of spindle and epithelioid cells of moderate to high nuclear grade; small biopsies of metastatic melanomas may be amelanotic (27). However, a vasoformative pattern of growth has not been described, and the reactivity of melanoma to antibodies to S-100 protein, HMB45, and MelanA 103 protein is dissimilar to the IHS profile of angiosarcoma.
Kaposi's sarcoma differs from metastatic angiosarcoma in that it usually is not as nodular, does not show intravascular growth (growth is primarily perivascular), and is not as cytologically atypical (9, 25). Pulmonary artery sarcoma, with an incidence one-fifth that of primary cardiac sarcomas, is extremely rare and usually lacks the hemorrhage and necrosis of metastatic angiosarcoma (9). The histologic appearance of pulmonary artery sarcoma varies and encompasses malignant bone and cartilage formation, for example, so corresponding immunohistochemical results may reveal positivity for a greater array of antigens than are seen in angiosarcomas (25, 28). Most commonly, pulmonary artery sarcoma is composed of fibroblastic or myofibroblastic malignant spindle cells; angiosarcomatous differentiation is rare (28).
Epithelioid hemangioendothelioma (EHE, previously termed intravascular bronchoalveolar tumor) is a low-grade sclerosing angiosarcoma. It most commonly occurs in women under the age of 40 (25). Like high-grade metastatic angiosarcoma, EHE often forms multiple bilateral nodules in the lungs. However, grossly the nodules are much less hemorrhagic with a typical translucent, gray appearance resembling hyaline cartilage. Microscopically, the epithelioid hemangioendothelioma is composed of balls of cells embedded in a myxohyaline or myxochondroid matrix. Tumor cells are cytologically bland in contrast to high-grade angiosarcomas, and they contain well-defined cytoplasmic vacuoles, a feature seen less uniformly in higher grade angiosarcomas (25, 29). A few EHE may exhibit greater nuclear atypia and an unusually high mitotic rate; such cases may be difficult to distinguish from high-grade metastatic angiosarcoma (25).
Intravascular lymphomatosis, which comprises intravascular growth of a large-cell lymphoma, predominantly of B-cell lineage, also enters into the differential of metastatic angiosarcoma (9). The vessels involved are usually capillaries, whereas angiosarcomas may grow within a range of vessels, usually larger in caliber (25). Lymphoma cells remain confined within the vessels, whereas a heterogeneous mixture of growth patterns is common in angiosarcomas. Interestingly, there may exist a rare form of angiosarcoma that is entirely intravascular, a true neoplastic angioendotheliomatosis. Lin and coworkers (30) recently reported two cases in which the tumor was intravascular, in multiple organs including the lungs, and comprised tumor cells reactive for at least two endothelial markers. Therefore, even though the far likelier diagnosis for an exclusive intravascular population of atypical cells is lymphoma, carcinoma, or melanoma, immunohistochemical evaluation of such lesions should include endothelial markers if there is no IHS reactivity characteristic of the more likely causes. In cases of intravascular lymphomatosis, IHS will reveal the hematopoietic nature of the malignant cells, with staining for B-cell markers and panleukocyte markers such as CD19, CD20 and leukocyte common antigen (LCA; 31).
CONCLUSION
In summary, this study of 21 extracutaneous angiosarcomas metastatic to the lungs indicates that the pathologist may be the first physician to render the diagnosis. The heart and pericardium are common sites of origin, with symptoms that are often not site specific or are mistaken for an inflammatory process. Many patients first present with lung-related symptoms. At the time of lung biopsy, 11 patients had no known primary site of origin, and angiosarcoma was not a clinical consideration. The radiologic features most commonly comprise multiple peripheral lung nodules suggestive of metastatic tumor, but a variety of patterns may occur. This also leads to a broad clinical etiologic differential. Open-lung biopsy is often required to make what is commonly an unsuspected diagnosis.
References
Meis-Kindblom JM, Kindblom L-G . Angiosarcoma of soft tissue: a study of 80 cases. Am J Surg Pathol 1998; 22: 683–697.
Suster S, Moran CA . Unusual manifestations of metastatic tumors to the lungs. Semin Diagn Pathol 1995; 12: 193–206.
Patel AM, Ryu JH . Angiosarcoma in the lung. Chest 1993; 103: 1531–1535.
Naka N, Ohsawa M, Tomita Y, et al. Angiosarcoma in Japan. A review of 99 cases. Cancer 1995; 75: 989–996.
Fukunaga M, Shimoda T, Nikaido T, Ushigome S, Ishikawa E . Soft tissue vascular tumors: a flow cytometric analysis. Cancer 1993; 71: 2233–2241.
Aust MR, Olsen KD, Meland NB, et al. Angiosarcomas of the head and neck: clinical and pathologic characteristics. Annu Otol Rhinol Laryngol 1997; 106: 943–951.
Yousem SA . Angiosarcoma presenting in the lung. Arch Pathol Lab Med 1986; 110: 112–115.
Sheppard MN, Hansell DM, Du Bois RM, Nicholson AG . Primary epithelioid angiosarcoma of the lung presenting as pulmonary hemorrhage. Hum Pathol 1997; 27: 383–385.
Colby TV . Malignancies in the lung and pleura mimicking benign processes. Semin Diagn Pathol 1995; 12: 30–44.
Raaf HN, Raff JH . Sarcomas related to the heart and vasculature. Semin Surg Oncol 1994; 10: 374–382.
Basso C, Valente M, Poletti A, Casarotto D, Thiene G . Surgical pathology of primary cardiac and pericardial tumors. Eur J Cardiothorac Surg 1997; 12: 730–738.
Donsbeck A-V, Ranchere D, Coindre J-M, Le Gall F, Cordier J-F, Loire R . Primary cardiac sarcomas: an immunohistochemical and grading study with long-term follow-up of 24 cases. Histopathology 1999; 34: 295–304.
Rodriguez A . Angiosarcomas of the interatrial septum mimicking atrial myxomas. J Am Soc Echocardiogr 1996; 9: 209–212.
Afzal MN . Primary cardiac angiosarcoma: clinical, pathological, and diagnostic problems. Can J Cardiol 1997; 13: 293–296.
Mullick SS, Mody DR, Schwartz MR . Angiosarcoma at unusual sites: a report of two cases with aspiration cytology and diagnostic pitfalls. Acta Cytol 1997; 41: 839–844.
Naka N, Ohsawa M, Tomita Y, et al. Prognostic factors in angiosarcoma: a multivariate analysis of 55 cases. J Surg Oncol 1996; 61: 170–176.
Mark RJ, Poen JC, Tran LM, Fu YS, Juillard GF . Angiosarcoma: a report of 67 patients and a review of the literature. Cancer 1996; 77: 2400–2406.
Nara M, Sasaki T, Shimura S, et al. Diffuse alveolar hemorrhage caused by lung metastasis of ovarian angiosarcoma. Intern Med 1996; 35: 653–656.
Miettenin M, Lindemayer AE, Chaubal A . Endothelial cell markers CD31, CD34, and BNH9 antibody to H- and Y- antigens: evaluation of their specificity and sensitivity in the diagnosis of vascular tumors and comparison with von Willebrand factor. Mod Pathol 1994; 7: 82–90.
Ohsawa M, Naka N, Tomita Y, Kawamori D, Kanno H, Aozasa K . Use of immunohistochemical procedures in diagnosing angiosarcoma. Cancer 1995; 75: 2867–2874.
Mills SE, Gaffey MJ, Watts JC, et al. Angiomatoid carcinoma and ‘angiosarcoma’ of the thyroid gland: a spectrum of endothelial differentiation. Am J Clin Pathol 1994; 102: 322–330.
Poblet E, Gonzalez-Palacios F, Jimenez FJ . Different immunoreactivity of endothelial markers in well and poorly differentiated areas of angiosarcomas. Virchows Arch 1996; 428: 217–221.
Ramani P, Shah A . Lymphangiomatosis—histologic and immunohistochemical analysis of four cases. Am J Surg Pathol 1993; 17: 329–335.
Appleton MAC, Attanoos RL, Jasani B . Thrombomodulin as a marker of vascular and lymphatic tumours. Histopathology 1996; 29: 153–157.
Colby TV, Koss MN, Travis WD . Tumors of the lower respiratory tract.In: Rosai J, editor. Atlas of tumor pathology. 3rd series, Fascicle 13. Washington, DC: Armed Forces Institute of Pathology; 1995.p. 361–363.
Ritter JH, Mills SE, Nappi O, Wick MR . Angiosarcoma-like neoplasms of epithelial origins: true endothelial tumors or variants of carcinoma? Semin Diagn Pathol 1995; 12: 270–282.
Nakhleh RE, Wick MR, Rocamora A, Swanson PE, Dehner LP . Morphologic diversity in malignant melanomas. Am J Clin Pathol 1990; 93: 731–740.
Burke A, Virmani R . Tumors of the heart and great vessels.In: Rosai J, editor. Atlas of tumor pathology. 3rd series, Fascicle 16. Washington, DC: Armed Forces Institute of Pathology; 1996.p. 217–221.
Enzinger FM, Weiss SW . Soft tissue tumors. 3rd ed. St. Louis, MO: Mosby Year-Book; 1995.p. 627–632.
Lin BT-Y, Weiss LM, Battifora H . Intravascularly disseminated angiosarcoma: true neoplastic angioendotheliomatosis? Am J Surg Pathol 1997; 21: 1138–1143.
Mann RB . Are there site-specific differences among extranodal aggressive B-cell neoplasms? Am J Clin Pathol 1999; 111(1 Suppl): S144–S150.
Acknowledgements
The authors thank Ms. Heather Pratt, M.S., University of New Mexico, Department of Pathology, Albuquerque, New Mexico, and Dr. I. Bos, University of Lubeck, Institute for Pathology, Lubeck, Germany, for assistance in obtaining patient follow-up information.
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Bocklage, T., Leslie, K., Yousem, S. et al. Extracutaneous Angiosarcomas Metastatic to the Lungs: Clinical and Pathologic Features of Twenty-One Cases. Mod Pathol 14, 1216–1225 (2001). https://doi.org/10.1038/modpathol.3880463
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3880463
Keywords
This article is cited by
-
A case of rapid progression of a cardiac tumor originating from the coronary sinus observed by transthoracic echocardiography
Journal of Medical Ultrasonics (2020)
-
Primary pulmonary artery sarcoma masquerading as pulmonary thromboembolism: a rare diagnosis unveiled
Clinical Sarcoma Research (2017)
-
The lung metastatic niche
Journal of Molecular Medicine (2015)
-
Application of immunocytochemistry to the diagnosis of primary epithelioid angiosarcoma of the lung
Medical Molecular Morphology (2009)
-
Metastatic angiosarcoma of the lung with alveolar hemorrhage
Japanese Journal of Radiology (2009)