Abstract
Mucoepidermoid carcinoma is a rare primary thyroid tumor with indolent biologic potential. Two types of tumors have been described under this category: mucoepidermoid carcinoma (MEC) and sclerosing mucoepidermoid carcinoma with eosinophilia (SMECE). The MEC shows both squamous and glandular differentiation in a background of a noninflamed gland, whereas SMECE is characterized by extensive sclerosis, squamous and glandular differentiation, a concomitant inflammatory infiltrate rich in eosinophils, and a background of lymphocytic thyroiditis.
We present nine cases of these entities: five MEC and four SMECE. All tumors occurred in women (age 27 to 73 years). Five tumors showed extrathyroidal invasion and multiple lymph node metastases. One case of MEC showed a concomitant tall cell variant of papillary carcinoma with vascular invasion, and two cases showed intimately associated areas of usual papillary carcinoma. One of the latter cases also showed areas of transformation to anaplastic carcinoma. In all cases of SMECE and in only one case of MEC, the uninvolved thyroid tissue showed lymphocytic thyroiditis. Follow-up information was available in four of the nine cases (3 months to 7 years). Two patients with SMECE are alive with no evidence of disease. One patient with MEC and tall cell variant of papillary carcinoma died of disease after 3 months, and the patient with anaplastic carcinoma died after 5 months with lung metastasis.
Both MEC and SMECE were positive for cytokeratin and negative for calcitonin. All cases of MEC were positive for thyroglobulin, whereas all cases of SMECE were negative. The immunohistochemical findings suggest that both MEC and SMECE have different histogenesis.
Similar content being viewed by others
INTRODUCTION
Mucoepidermoid carcinoma (MEC) is most commonly encountered in salivary glands but can also be seen at other locations, including bronchus, trachea, esophagus, breast, pancreas, and thyroid gland (1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25). MEC of the thyroid gland is a rare tumor and is characterized by unique histologic appearance and indolent biologic behavior (10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25). Two types of tumors have been described under this heading: MEC (10, 11, 12, 13, 14, 15, 16, 17, 18) and sclerosing mucoepidermoid carcinoma with eosinophilia (SMECE) (19, 20, 21, 22, 23, 24, 25). There is interesting debate in the literature regarding the histogenesis of these tumors (19, 20, 21, 26, 27, 28, 29). Many authors have suggested that these tumors develop from ultimobranchial body rests/solid cell nests (19, 26, 27, 28, 29), whereas some authors have reported a follicular origin (14, 16, 17, 20). The other suggested sources include C cells, parathyroid, ectopic salivary gland, and thyroglossal duct (10, 11, 16).
Fewer than 30 cases of these entities have been described in the literature. We report clinicopathologic features of eight additional cases (five MEC and four SMECE cases) and discuss various proposed theories of their cell of origin in light of our findings.
MATERIALS AND METHODS
Five cases of primary MEC and three cases of SMECE were retrieved from the consultation files of one of the authors (VAL), and one additional case of SMECE was retrieved from the surgical pathology files of University of Pennsylvania Medical Center. Hematoxylin and eosin–stained slides were reviewed in all cases; unstained paraffin sections were used for immunohistochemical analysis. The avidin-biotin-peroxidase complex technique was used for immunostaining. The antibody panel included antithyroglobulin, calcitonin, and cytokeratins (AE1/AE3) (Dako, Carpinteria CA).
RESULTS
The main clinicopathologic findings are summarized in Table 1. For the purposes of this article, we divided the tumors into two separate entities, MEC and SMECE, on the basis of pathologic (light microscopy and immunohistochemistry) findings.
Mucoepidermoid Carcinoma
Two patients were men, and three were women, ranging in age from 27 to 83 years. The clinical information was available in four cases, which was that of a painless unilateral thyroid mass. Radiographic studies showed a cold nodule. Two patients underwent partial thyroidectomy, and two had total thyroidectomy. Follow-up information was available in three cases; two patients died of disease and one is alive with no evidence of disease.
Pathologic findings
Gross pathology findings were available in three cases, which showed a well-circumscribed mass ranging in size from 1.6 to 10 cm. On cut sections, the tumors were tan-brown in color and showed solid and cystic areas.
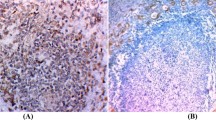
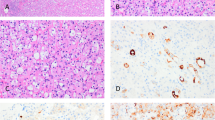
By light microscopy, all cases revealed notable areas of both epidermoid and duct-like elements. The epidermoid elements were arranged in solid sheets with keratin pearl formation. Glandular spaces filled with mucinous material forming mucous cysts were noted in three cases; mucous cells lined the ductal elements (Figs. 1 and 2A). Mucicarmine stain highlighted the mucinous material present in areas of glandular differentiation (Fig. 2B). The background stroma showed varying degrees of fibrosis and foci of psammomatous calcification. Two cases showed a concomitant aggressive thyroid tumor; one case showed an associated tall cell variant of papillary carcinoma (Fig. 3), and the other showed an anaplastic carcinoma with extensive involvement of the normal thyroid parenchyma and perithyroidal tissues. One case showed lymphocytic thyroiditis.
By immunohistochemistry, all cases were positive for thyroglobulin and cytokeratin and negative for calcitonin. In a case with a concomitant anaplastic carcinoma, the MEC showed positive staining with thyroglobulin, whereas the anaplastic tumor was negative.
Sclerosing Mucoepidermoid Carcinoma with Eosinophilia
All patients were women, who ranged in age from 38 to 73 years. All tumors were in the right thyroid and presented as a solitary mass that was cold on scan. Three patients underwent partial thyroidectomy, and one had total thyroidectomy and lymph node dissection. Follow-up information was available in two cases; both are alive without any evidence of disease.
Pathologic findings
Grossly, all tumors were circumscribed and ranged in size from 3 to 6 cm. Three cases showed predominantly solid tumor, and one revealed a 1-cm cystic area within a 3-cm solid tumor. Only one case revealed extrathyroidal extension into the skeletal muscle, whereas in the remaining three cases the tumors were confined to the thyroid gland. Follow-up information was available in two cases; both are alive without any evidence of disease.
Microscopically, in all cases, the non-neoplastic thyroid parenchyma showed lymphocytic thyroiditis. The tumor cells were arranged in small islands, anastomosing cords, and narrow strands and displayed prominent squamous differentiation characterized by intercellular bridges and keratin pearl formation. Glandular structures resembling mucous cysts were noted in two cases (Fig. 4). The background stroma in the main tumor mass revealed marked sclerosis and a mixed inflammatory infiltrate with prominent eosinophilia (Fig. 4B). Lymphatic permeation was identified in one case with extensive lymph node involvement. Two cases revealed a separate focus of follicular variant of papillary thyroid carcinoma and papillary microcarcinoma. Both these lesions were present in the same lobe away from the main tumor mass.
Sclerosing mucoepidermoid carcinoma with eosinophilia (SMECE). A, tumor cells arranged in cords and nests in a background of sclerosis and lymphocytic thyroiditis. B, another view of SMECE showing tumor nests with mucous cyst formation in a background of sclerosis. C, high-power view showing tumor nest in a background eosinophil-rich (arrow) inflammatory infiltrate. D, squamous and glandular differentiation. E, a positive mucicarmine stain highlighting the mucin production in SMECE.
The tumor cells stained negative for thyroglobulin and calcitonin and positive for cytokeratin (AE1/AE3) in all cases. Mucin stain was positive in one case with mucous cysts (Fig. 4E).
DISCUSSION
MEC is most commonly encountered as a tumor of salivary glands; it has been described in the major salivary glands and in the minor glands, particularly in the oral cavity (1). Bronchial origin is unusual, but probably this tumor is the most frequent after carcinoid (2, 3).
The MEC occurs anywhere in the thyroid gland and appears as a partly circumscribed lesion, generally soft in consistency. It is most often found in female patients; the female-to-male ratio is 16:9 (14, 15, 20). The ages of the patients range from 10 to 66 years. Histologically, it bears resemblance to the lesion of the salivary glands, showing mucin-containing cysts of varying size and solid epithelial nests (10, 15, 20). Intracytoplasmic mucin (as stained by mucicarmine and alcian blue stains) can be found especially in tumor cells lining the cysts (10, 11, 12, 13, 14, 15, 16, 17, 18). Associated foci of recognizable papillary carcinoma may form part of the tumor (17). Separate foci of usual papillary carcinoma or follicular variant, usually microscopic in size, can be seen in the thyroid removed from the main mass (10, 11, 12, 13, 14, 15, 16, 17). The background thyroid usually is normal, and thyroiditis is not common (10, 11, 12, 13, 14, 15, 16, 17, 18).
The sclerosing mucoepidermoid carcinoma is a distinctive tumor that bears no resemblance to any of the usual epithelial thyroid neoplasms. It is a tumor of adults and occurs in patients between 35 and 71 years of age (19). It is overwhelmingly a tumor of women (female:male ratio, 17:1) (19, 20). It is a diffusely infiltrating tumor, which is usually located in the lateral aspects of the gland. The tumor consists of nests of epidermoid or even squamous appearance (keratinization is rare but does occur) and mucin-containing cysts. The latter may be numerous or very rare. There is extensive fibrosis associated with the tumor, and this stroma is replete with eosinophilic leukocytes (19). The background thyroid is always affected by chronic lymphocytic thyroiditis, frequently with extensive fibrosis (19, 20, 21).
Both MEC and SMECE should be differentiated from thyroid tumors that can show foci of squamous differentiation and primary squamous carcinoma of thyroid (26, 27, 28, 29, 30, 31, 32). The list of thyroid tumors with areas of squamous change includes papillary carcinoma and its variants, medullary carcinoma, carcinoma with thymus-like differentiation, anaplastic carcinoma, and rarely follicular adenoma and carcinoma (26, 27, 28, 29, 30, 31, 32). Papillary carcinoma frequently shows areas of squamous change; however, it can be distinguished from other tumors by its characteristic nuclear morphology and its positivity for thyroglobulin and negativity for calcitonin stains (26, 27). Medullary carcinoma can also exhibit areas of squamous and mucinous change (26, 27, 28, 29, 30) and along with its sclerotic amyloid-rich stroma can mimic SMECE. However, lack of lymphocytic thyroiditis and positive calcitonin stain will differentiate medullary carcinoma from SMECE. Squamous cell carcinoma of the thyroid is a very rare tumor and portends a poor prognosis. These tumors usually are seen in older patients who have a history of goiter. These morphologically resemble squamous cell carcinomas of other organs and range from well- to poorly differentiated lesions (31, 32).
MEC and SMECE differ in their immunoprofile in that the MEC is frequently thyroglobulin positive, whereas the SMECE is negative for thyroglobulin and both tumors are negative for calcitonin (19, 20). The immunophenotype of the latter tumor and its association with chronic thyroiditis suggest origin from rests of ultimobranchial body or solid cell nests (19, 33, 34, 35, 36). These structures are often hyperplastic in severe chronic thyroiditis and are located in the lateral lobes of the thyroid. Hence, the possibility of a spectrum of hyperplasia to neoplasia of ultimobranchial body rests should be considered for the SMECE. However, the MEC by its immunophenotype, location in the gland, and association with papillary thyroid carcinoma suggests that it may be a variant of usual papillary thyroid carcinoma derived from follicular epithelial cells (10, 11, 12, 13, 14, 15, 16, 17, 18, 20).
The prognosis of these two tumors is similar in that they seem to represent indolent malignancies (10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23, 24, 25). The MEC can give rise to nodal metastases and rarely spreads beyond the neck. Mortality from this tumor is unusual but has been reported (10, 11, 12, 13, 16). In keeping with the hypothesis that this tumor is a follicular-derived neoplasm, one of our cases developed an anaplastic carcinoma that led to the death of the patient. In two previously reported cases (10, 16), anaplastic carcinoma was also found and both of these patients died of their tumor.
The SMECE can behave in a slowly growing manner and even in the presence of extension outside the thyroid can be associated with prolonged survival. Rare cases of lung and other distant metastases are reported (21, 22). The eosinophilia, which is an important diagnostic clue to this lesion, remains unexplained (19, 21, 22, 23, 24, 25), but it is likely that the tumor produces a cytokine that serves as a chemo-attractant to eosinophils. Whether this feature is responsible for the indolent behavior of this lesion even in cases with extrathyroidal extension remains to be determined.
On the basis of the available literature and our experience reported here, we therefore suggest that there are two histologically distinctive tumors that have been placed in the diagnostic category of MEC of the thyroid gland. The first is the tumor that most resembles a carcinoma of salivary gland origin and is a form of usual papillary cancer that happens to have acquired the ability to produce mucin. The second is a non–follicular-derived tumor of probable ultimobranchial origin, which occurs in the setting of chronic thyroiditis and does not share its histologic appearance with that of the salivary tumor that bears a similar nomenclature.
References
Eversole LR . Mucoepidermoid carcinoma. Review of 815 reported cases. J Oral Surg 1970; 28: 490–494.
Klacsmann PG, Olson JL, Eggleston JC . Mucoepidermoid carcinoma of the bronchus. Cancer 1968; 43: 1053–1059.
Green L, Gallion T, Gyorkey F . Peripheral mucoepidermoid tumors of the lung. Thorax 1991; 46: 65–66.
Kay S . Mucoepidermoid carcinoma of the esophagus: report of two cases. Endoscopy 1968; 22: 1053–1059.
Hartsup N, Sehested M . High-grade mucoepidermoid carcinoma of the breast. Histopathology 1985; 9: 887–892.
Markopoulos C, Gogas H, Livaditou A, Floros D . Mucoepidermoid carcinoma of the breast. Eur J Gynecol Oncol 1998; 19: 291–293.
Tomita T, Lotuaco L, Talbott L, Watanabe I . Mucoepidermoid carcinoma of the subglottis. Arch Pathol Lab Med 1977; 101: 145–148.
Ohtsuki Y, Yoshino T, Takahashi K, Sonobe H, Kohno K, Agaki T . Electron microscopic study of mucoepidermoid carcinoma in the pancreas. Acta Pathol Jpn 1987; 37: 1175–1182.
Onoda N, Kang SM, Sugano S, Yamashita Y, Chung YS, Sowa M . Mucoepidermoid carcinoma of the pancreas: report of a case. Surg Today 1995; 25: 843–847.
Fransilla KO, Harach HR, Wasenius VM . Mucoepidermoid carcinoma of the thyroid. Histopathology 1984; 8: 847–860.
Rhatigan RM, Rouque JL, Bucher RL . Mucoepidermoid carcinoma of the thyroid gland. Cancer 1977; 39: 210–214.
Mizukami Y, Matsubara F, Hashimoto T, Haratake J, Terahata S, Noguchi M, et al. Primary mucoepidermoid carcinoma in the thyroid gland. A case report including an ultrastructural and biochemical study. Cancer 1984; 53: 1741–1745.
Larson RS, Wick MR . Primary mucoepidermoid carcinoma of the thyroid: diagnosis by fine-needle aspiration biopsy. Diagn Cytopathol 1993; 9: 438–443.
Arezzo A, Patetta R, Ceppa P, Borgonovo G, Torre G, Mattioli FP . Mucoepidermoid carcinoma of the thyroid gland arising from a papillary epithelial neoplasm. Am Surg 1998; 64: 307–311.
Viciana MJ, Galera-Davidson H, Martin-Lacave, Segura DI, Loizaga JM . Papillary carcinoma of the thyroid with mucoepidermoid differentiation. Arch Path Lab Med 1996; 120: 397–398.
Cameselle-Teijeiro J, Febles-Perez C, Sobrinho-Simoes M . Papillary and mucoepidermoid carcinoma of the thyroid with anaplastic transformation: a case report with histologic and immunohistochemical findings that support a provocative histogenetic hypothesis. Path Res Pract 1995; 191: 1214–1221.
Miranda RN, Myint M, Gnepp DR . Composite follicular variant of papillary carcinoma and mucoepidermoid carcinoma of the thyroid. Report of a case and review of the literature. Am J Surg Pathol 1995; 19: 1209–1215.
Katoh R, Sugai T, Ono S, Takayama K, Tomichi N, Kurihara H, et al. Mucoepidermoid carcinoma of the thyroid gland. Cancer 1990; 65: 2020–2027.
Chan JK, Albores-Saavedra J, Battifora H, Carcangui ML, Rosai J . Sclerosing mucoepidermoid thyroid carcinoma with eosinophilia. A distinctive low-grade malignancy arising from the metaplastic follicles of Hashimoto’s thyroiditis. Am J Surg Pathol 1991; 15: 438–448.
Wenig BM, Adair CF, Heffess CS . Primary mucoepidermoid carcinoma of the thyroid gland: a report of six cases and a review of the literature of follicular epithelial-derived tumor. Hum Pathol 1995; 26: 1099–1108.
Sim SJ, Ro JY, Ordonez NG, Cleary KR, Ayala AG . Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid: report of two patients, one with distant metastasis, and review of the literature. Hum Pathol 1997; 28: 1091–1096.
Geisinger KR, Steffee CH, McGee RS, Woodruff RD, Buss DH . The cytomorphologic features of sclerosing mucoepidermoid carcinoma of the thyroid gland with eosinophilia. Am J Clin Pathol 1999; 111: 134–136.
Cavazza A, Toschi E, Valcavi R, Piana S, Scotti R, Carlinfante G, et al. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid: description of a case. Pathologica 1999; 91: 31–35.
Chung J, Lee SK, Gong G, Kang DY, Park JH, Kim SB, et al. Sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid glands: a case report with clinical manifestation of recurrent neck mass. J Korean Med Sci 1999; 14: 338–341.
Solomon AC, Baloch ZW, Salhany KE, Mandel S, Weber RS, LiVolsi VA . Thyroid sclerosing mucoepidermoid carcinoma with eosinophilia: mimic of Hodgkin’s disease in nodal metastases. Arch Pathol Lab Med 2000; 124; 446–449.
LiVolsi VA . Surgical pathology of the thyroid. Philadelphia: WB Saunders; 1990.
Rosai J, Carcangui ML, DeLellis RA . Tumors of the thyroid gland. Atlas of tumor pathology. 3rd Series, Fascicle 5. Washington, DC: Armed Forces Institute of Pathology; 1992.
Dominguez-Malagon H, Delgado-Chavez R, Torres-Najera M, Gould E, Albores-Saavedra J . Oxyphil and squamous variants of medullary thyroid carcinoma. Cancer 1989; 63: 1183–1188.
Schmid KW, Tötsch M, Hittmair A, Feichtinger H, Ladurner D, Hofstäder F . Frequency of medullary thyroid carcinoma in an endemic goiter area. Mod Pathol 1989; 2: 90–93.
Schröder S, Böcker W, Baisch H, Bürl CG, Arps H, Meiners I, et al. Prognostic factors in medullary thyroid carcinomas. Survival in relation to age, sex, stage, histology, immunocytochemistry, and DNA content. Cancer 1988; 61: 806–816.
Huang TY, Assor D . Primary squamous cell carcinoma of the thyroid gland. Am J Clin Pathol 1971; 55: 93–98.
Cook AM, Vini L, Harmer C . Squamous cell carcinoma of the thyroid: outcome of treatment in 16 patients. Eur J Surg Oncol 1999; 25: 606–609.
Ozaki O, Ito K, Sugino K, Yasuda K, Yamashita T, Toshima K . Solid cell nests of the thyroid gland: precursor of mucoepidermoid carcinoma. World J Surg 1993; 17: 417–418.
Harach HR . A study on the relationship between solid cell nests and mucoepidermoid carcinoma of the thyroid. Histopathology 1985; 9: 195–207.
Harach HR, Vujanic GM, Jasani B . Ultimobranchial body rests in human fetal thyroid: an autopsy, histological, and immunohistochemical study in relation to solid cells nests and mucoepidermoid carcinoma of the thyroid. J Pathol 1993; 169: 465–469.
Harach HR . Histological markers of solid cell nests of the thyroid. With some emphasis on their expression in thyroid ultimobranchial related tumors. Acta Anat (Basel) 1985; 124: 111–116.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Baloch, Z., Solomon, A. & LiVolsi, V. Primary Mucoepidermoid Carcinoma and Sclerosing Mucoepidermoid Carcinoma with Eosinophilia of the Thyroid Gland: A Report of Nine Cases. Mod Pathol 13, 802–807 (2000). https://doi.org/10.1038/modpathol.3880140
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3880140
Keywords
This article is cited by
-
NSD3-NUTM1-rearranged carcinoma of the median neck/thyroid bed developing after recent thyroidectomy for sclerosing mucoepidermoid carcinoma with eosinophilia: report of an extraordinary case
Virchows Archiv (2021)
-
Salivary-Like Tumors of the Thyroid: A Comprehensive Review of Three Rare Carcinomas
Head and Neck Pathology (2021)
-
Sclerosing mucoepidermoid carcinoma of the salivary glands: report of three cases with special concern to the counterpart accompanied by eosinophilia
Medical Molecular Morphology (2021)
-
Exploring the molecular insights of concurrent composite mucoepidermoid carcinoma and papillary thyroid carcinoma
Endocrine (2020)
-
Activating BRAF mutation in sclerosing mucoepidermoid carcinoma with eosinophilia of the thyroid gland: two case reports and review of the literature
Journal of Medical Case Reports (2019)