Abstract
The clinicopathologic features of 48 tumors that were histologically similar to gastrointestinal stromal tumors but occurred in the soft tissues of the abdomen were analyzed to determine their overall similarity to their gastrointestinal counterpart, their biologic behavior, and the parameters that predict risk for adverse outcome. Classic leiomyomas and leiomyosarcomas were specifically excluded. The tumors occurred in 32 women and 16 men, who ranged in age from 31 to 82 years (mean, 58 years). Forty tumors arose from the soft tissue of the abdominal cavity, and the remainder arose from the retroperitoneum. They ranged in size from 2.1 to 32.0 cm and varied from tumors composed purely of rounded epithelioid cells to those composed of short fusiform cells set in a fine fibrillary collagenous background with some cases showing a mixed pattern. Tumors displayed variable amounts of stromal hyalinization, myxoid change, and cyst formation. The tumors expressed CD117 (c-kit receptor) (100%), CD34 (50%), neuron-specific enolase (44%), smooth muscle actin (26%), desmin (4%), and S-100 protein (4%). Tumors were evaluated with respect to several parameters: size (<10 cm or >10 cm), cellularity (low or high), mitoses (0 to 2 per 50 high-power fields, >2 per 50 high-power fields), nuclear atypia (1 to 3+), cell type (epithelioid, spindled, or mixed), and necrosis (absent or present). These parameters were then evaluated in univariate and multivariate analysis with respect to adverse or nonadverse outcome, the former defined as metastasis or death from tumor. Follow-up information was obtained for 31 patients (range, 4 to 84 months; median, 24 months). One patient presented with an adverse event and, therefore, was excluded from subsequent analysis. Twelve patients (39%) developed metastases or died of tumor. In univariate analyses, cellularity, mitotic activity (>2 per 50 high-power fields), and necrosis were associated with statistically significant increases in the risk for adverse outcome. Despite the relatively small sample size, in a multivariable analysis mitotic activity (relative risk, 7.46; P =.09) and necrosis (relative risk, 3.75; P =.07) displayed trends toward independent predictive value. No association was noted between histologic pattern and outcome. Although only 39% of tumors behaved in a malignant fashion, this figure probably represents a conservative estimate because long-term follow-up (>5 years) was available for only a limited number of patients. Stratification of patients who have extragastrointestinal stromal tumor into those with 0 to 1 adverse histologic factors versus those with 2 to 3 offers the advantage of separating patients into two groups that have a markedly different risk for adverse outcome in the short term (0.02 events versus 0.54 events per person-year; P<.001, respectively). Extragastrointestinal (soft tissue) stromal tumors are histologically and immunophenotypically similar to their gastrointestinal counterpart but have an aggressive course more akin to small intestinal than gastric stromal tumors.
Similar content being viewed by others
INTRODUCTION
Mesenchymal tumors that arise from the wall of the gastrointestinal tract have attracted a great deal of attention over the years. It is generally accepted that those that arise from the esophagus and rectum recapitulate the appearance of mature smooth muscle (1, 2, 3), whereas the vast majority of tumors that arise from the remainder of the gastrointestinal tract show little or no differentiation along myocyte lines. Consonant with these observations, Stout (4) proposed the term leiomyoblastoma in 1960 for gastrointestinal lesions that have an epithelioid or round cell appearance and suggested that they were derived from an immature smooth muscle cell. Unfortunately, the former term implied little about biologic behavior, making a large body of the early literature difficult to interpret. With time, the terms epithelioid leiomyoma and epithelioid leiomyosarcoma supplanted the term leiomyoblastoma not only for such lesions in the gastrointestinal tract but also for analogous lesions in soft tissue (5, 6, 7). In 1983, Mazur and Clark (8) published a seminal paper documenting the absence of muscle markers within the majority of these lesions and the unexpected finding of neural markers in some by immunohistochemistry. They postulated that these lesions display various lines of differentiation reflecting the various elements of the gut wall (e.g., muscle, autonomic nerve) and proposed the term (gastrointestinal) stromal tumor (GIST). Over the past few years, it has become increasingly apparent that these tumors need to be studied on a site-specific basis because of differences in behavior (2, 9) and that malignancy is best described in terms of risk factors (10). Although numerous studies have addressed these issues in GIST (11, 12, 13, 14, 15, 16, 17, 18, 19, 20), there has been no study of those rare stromal tumors that develop outside the gastrointestinal tract in the soft tissues of the abdomen with respect to histologic features that predict outcome. A recent study compared a small number of omental and mesenteric GIST with respect to outcome but did not analyze various histologic parameters within these two groups (13). This study analyzes the clinicopathologic features of 48 such cases and evaluates a number of parameters to determine which most accurately predict adverse outcome. For purposes of this study, we refer to these lesions as extragastrointestinal stromal tumor (EGIST) and excluded lesions with classic features of leiomyoma and leiomyosarcoma.
MATERIALS AND METHODS
Cases used in this study were culled from one author's personal consultation files (SWW) and from the files of the Cleveland Clinic Foundation and were retrieved by searching all cases in the abdominal cavity or retroperitoneum which had been previously diagnosed as epithelioid leiomyoma, epithelioid leiomyosarcoma, leiomyoblastoma, or epithelioid smooth muscle tumor. The criteria for inclusion were relatively stringent to segregate a pure group of soft tissue lesions as opposed to a group having an admixture of gastrointestinal tumors. All tumors that arose from the gastrointestinal tract were excluded, including tumors that were attached to the serosa of the gastrointestinal tract on the grounds that they represented subserosal mural lesions. In addition, cases in which the origin of the mass was not clearly described in the intraoperative or gross report were also excluded on the premise that such lesions might also be gastrointestinal in origin. Gross descriptions and hematoxylin and eosin–stained slides were reviewed in each case. Follow-up information was obtained in 31 cases.
Each case was independently reviewed by three pathologists (JDR, JRG, SWW). A number of parameters were evaluated in each case. They included site, size, predominant cell type (spindled, epithelioid, or mixed), cellularity (low or high), nuclear pleomorphism (1, 2, 3), mitotic activity (0 to 2 mitoses/50 high-power fields [HPF] and >2 mitoses/50 HPF), and coagulative necrosis. Selection of a threshold level of more than 2 mitoses/50 HPF was somewhat arbitrary. However, selection of a low level of mitotic activity serves to identify lesions that are essentially mitotically inactive, which in turn may prove to be a reliable indicator of behavior. Predominant cell type was defined as the pattern present in more than 50% of the tumor. Tumors classified as spindled consisted of spindled or fusiform cells usually associated with a fibrillary collagenous background, whereas epithelioid tumors consisted of relatively round cells. Tumors were classified as mixed when the proportions of the two patterns was roughly equal. Cellularity was considered high when nuclei frequently touched one another, whereas in low cellularity tumors the nuclei were clearly separate from one another. Mitotic activity was determined by counting the number of mitotic figures per 50 HPF in the area of highest cellularity. Coagulative necrosis was considered either present or absent but was not further quantitated. Coagulative (or ischemic) necrosis was defined as ghosted neoplastic cells sharply abutting viable ones.
Clinical information and follow-up information were obtained from referring pathologists and medical records. Patients were considered to have experienced an adverse outcome if they developed metastases and/or died as a complication of their tumor. Metastasis was defined as recurrent tumor at some distance from the original tumor or tumor recurring as multiple lesions. In the absence of these features, patients were not considered to have had an adverse outcome.
For the 31 individuals for whom we had follow-up information, patient and tumor-specific variables were related to the time to experience an adverse event. Univariate assessments were made via Kaplan-Meier plots and by means of the log-rank test. Variables that were significant univariately were subsequently included in a multivariable Cox regression model to assess the potential for independent predictive value. All such analyses were performed using the SAS commercial software package (SAS Institute, Inc., Cary, NC). Because the total number of individuals and observed events was relatively small, an exact test (21) was performed to supplement the initial analyses whereby potential risk factors for adverse events had been identified. This test assessed the difference in incidence rates of adverse events between individuals who had no or one of the potential risk factors and those who had two or more of the risk factors.
Immunohistochemistry was performed in 25 cases by the avidin-biotin peroxidase complex method. In some cases, less material was available and, therefore, antibodies directed against recent antigens (e.g., c-kit) was performed on fewer cases. Immunostains for the following antigens were performed: smooth muscle actin (1A4; Sigma, St. Louis, MO; 1:1600), desmin (D33; DAKO, Carpinteria, CA; 1:10), S-100 protein (DAKO; 1:8000), neuron-specific enolase (Biogenex, San Ramon, CA; 1:160), CD34 (Becton Dickinson, San Jose, CA; 1:20), c-kit (CD117; DAKO; 1:200), cytokeratin (AE1/3; DAKO1:50), and chromogranin (Ventana, Tucson, AZ). Tumors were considered positive if more than 10% of the cells stained for the antigen.
RESULTS
Clinical Findings
The patients included 32 women and 16 men whose ages ranged from 31 to 82 years (mean, 58 years). In most instances, patients presented with an enlarging abdominal mass of variable duration often accompanied by vague abdominal pain. In two cases, the lesions were discovered incidentally during a workup for unrelated conditions. Forty tumors arose within the abdominal cavity, where they involved the omentum or mesentery; the remaining 8 tumors were located in the retroperitoneum. Follow-up information was obtained for 31 patients (range of follow-up, 4 to 84 months; median, 24 months). Twelve patients (39%) had an adverse outcome; of these, 10 died of their tumor, whereas 2 developed metastasis but had not succumbed to tumor as of the last known follow-up (Table 1). One patient developed a recurrent tumor in the general area of the original tumor and was alive without evidence of disease at 7 years. This patient was considered to have a local recurrence as opposed to metastasis and was not relegated to the adverse outcome group.
Gross and Histologic Findings
The tumors ranged in size from 2.1 to 32 cm with a median size of 12 cm. Of the 31 patients for whom follow-up data were available, 11 had tumors that were 10 cm or less in diameter, and the remainder had tumors with diameter greater than 10 cm. In most cases, the tumors were nodular or multinodular masses with a tan, friable surface. Several tumors were described as grossly hemorrhagic or necrotic, and in some instances cystic cavities were present. In all cases in which a segment of bowel was resected, the tumor was described as grossly separate from the bowel wall; this was confirmed microscopically.
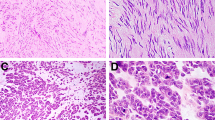
The histologic appearance of EGIST was variable, but, in general, two principal patterns were noted. Tumors could display predominantly one (44 cases) or a mixture of both (4 cases) patterns. The more common epithelioid pattern, observed in 24 cases and reminiscent of old descriptions of leiomyoblastoma, was characterized by uniform rounded cells with relatively scant cytoplasm arranged in sheets or dispersed singly throughout a finely or densely collagenized background (Fig. 1A, B). Occasionally, the epithelioid cells acquired a prominent cytoplasmic vacuole that in the extreme case resulted in formation of a signet ring cell (Fig. 1D). A less common change was degenerative nuclear atypia, which was cells that had one or more hyperchromatic nuclei sometimes arranged in a peripheral fashion (Fig. 1C). Such lesions typically were of low cellularity and devoid of mitotic activity. Highly cellular tumors, which proved to be associated with a high risk for adverse outcome, could be characterized either by large eosinophilic cells or by small round cells with a high nuclear cytoplasmic ratio (Fig. 2).
A, low-power view of epithelioid pattern in extragastrointestinal stromal tumor (EGIST). B, epithelioid pattern in EGIST of low cellularity demonstrating evenly dispersed round regular cells. C, EGIST showing cells with degenerative nuclear atypia and multinucleation. Tumors with this form of nuclear atypia are typically of low cellularity and have no mitotic activity. D, EGIST showing vacuolization of cells with formation of signet ring forms.
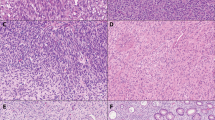
Twenty cases were classified as having a spindled pattern. These tumors more closely resembled a conventional smooth muscle than the epithelioid tumors. However, in contrast to cells of a conventional smooth muscle tumor, these cells were usually short and fusiform with a lightly staining cytoplasm (Fig. 3A–C). They usually were not arranged in long, well-oriented fascicles like a leiomyosarcoma but rather formed short or less defined fascicles. In a few, nuclear palisading was noted (Fig. 3D). Spindled tumor with high cellularity, associated with an increased risk for adverse outcome, were usually associated with increasing nuclear size and atypia as well as hemorrhage and necrosis (Fig. 3E, F).
A, low-power view of spindled pattern in extragastrointestinal stromal tumor (EGIST). Fascicles are short and ill-defined. B, high-power view of spindled EGIST of low cellularity showing short fusiform cells with little atypia. C, spindled EGIST showing areas with longer fascicles and closer resemblance to conventional leiomyosarcoma. D, spindled EGIST with nuclear palisading. E, spindled EGIST showing areas of low (benign appearing) and high (malignant appearing) cellularity. F, high-power view of high cellularity areas from E. These areas have increased nuclear atypia.
Several unusual or striking features were identified within EGIST of both types. Microscopic cysts, the result of collections of myxoid stroma within areas of tumor breakdown, was relatively common as a focal feature (Fig. 4A). Hyalinization both around vessels and within tumor was somewhat less common (Fig. 4B). Skeinoid fibers, which have been noted commonly within GIST of the small intestine, were not noted in our cases.
Immunohistochemical Findings
The results of the immunohistochemical findings are summarized in Table 2. All cases stained strongly for c-kit receptor in a majority of cells in a pattern that accentuated the cell borders (Fig. 5), whereas only 50% of cases were CD34 positive and usually more focally than c-kit.
Statistical Assessment of Histologic Variables as Risk Factors
Cellularity, mitotic activity, nuclear pleomorphism, and necrosis were evaluated independent of cell type in the 31 cases for which follow-up information was available and tabulated as to adverse versus nonadverse outcome, as described above (Table 3). Mitotic counts varied from 1 to 113 mitotic figures/50 HPF counted (mean, 13 mitotic figures). In univariate analyses, cellularity, mitotic activity, and necrosis all were significantly associated with outcome (P <.001 for each, log-rank test). Specifically, patients who had lesions that were highly cellular, had a high mitotic rate (>2/50 HPF), or had necrosis had significantly increased risk for experiencing an adverse outcome compared with patients who had lesions that did not have these features (Table 3). In a multivariable analysis, mitotic activity (relative risk, 7.46) and necrosis (relative risk, 3.75) showed trends toward independent prognostic value for adverse outcome, although statistical significance was not achieved at the 0.05 level (Table 4). The association between the number of adverse histologic factors (necrosis, cellularity, or mitotic activity) and the incidence of adverse outcome is shown in Table 5. Overall, 5% of patients who had no or one risk factor developed adverse outcomes, compared with 92% of patients who had two or three risk factors. The corresponding incidence rates were 0.02 events per person-year of follow-up, as opposed to 0.54 per person-year (P <.001).
DISCUSSION
Stromal tumors that arise outside the gastrointestinal tract are rare. Generally considered similar to their gastrointestinal counterpart, there have been no large studies dedicated specifically to EGIST to support this assumption. In fact, they are often included in large studies of stromal tumors in which they account for less than 10% of the overall group (22, 23). Thus, any differences in this subgroup, compared with those arising from or attached to the gut, would potentially be eclipsed. There is growing evidence that despite that virtually all stromal tumors express the c-kit receptor, they display various site-specific differences. Most important, the behavior of stromal tumors differs by location, and there seems to be a general trend for increasingly aggressive behavior as one proceeds distally along the gastrointestinal tract (11, 12, 16, 17, 18, 19). For example, the majority of GIST located in the stomach have a good prognosis, whereas those in the small intestine have a significantly worse prognosis. Nearly one half of our patients developed metastatic disease or died of tumor within a relatively brief follow-up period (mean, 2 years), indicating that these lesions are biologically more like those encountered in the distal gastrointestinal tract.
The major purpose of this study was to determine which parameters are important in determining the risk for adverse outcome; for that purpose, we analyzed parameters that arguably affect prognosis and that have been studied extensively in GIST. These included cellularity, mitotic activity, size, nuclear atypia, necrosis, and growth pattern (spindled versus epithelioid). As depicted in Table 3, cellularity, mitotic activity, and necrosis were significantly associated with adverse outcome in a univariate analysis, whereas nuclear atypia, growth pattern, and size were not. In a multivariable analysis, necrosis and mitotic activity showed trends toward independent predictive value, but likely these trends did not reach statistical significance at the 0.05 level, likely because of a small sample size. An exact test revealed that grouping patients in terms of the number of potential risk factors (zero or one of cellularity, mitotic activity, or necrosis versus two or three of these factors) was highly discriminatory as a diagnostic indicator of the likely incidence of an adverse event (P <.001).
Our findings are in many respects in agreement with a series of thematic studies that were analyzed in a similar fashion from one institution (12, 17, 18, 19) (Table 6). Cellularity and mitotic activity both are consistent predictors of aggressive behavior in the stomach, duodenum, jejunum/ileum, and colon, although neither has been shown to be statistically important in the rectum. Mitotic activity has been associated with adverse outcome in nearly all studies but is best viewed as a continuous variable in which threshold levels are selected for analysis depending on the site in question. We originally evaluated the effect of two levels of mitotic activity in our statistical model. At both a level of more than 2 mitoses/50 HPF (as has been used in the duodenum) and a level of more than 5 mitoses/50 HPF (commonly used in studies of GIST of the jejunum and ileum). statistical significance was achieved in a univariate but not in a multivariate analysis. However, use of the level of more than 2 mitoses/50 HPF results in a greater relative risk value for the group in a multivariate analysis and therefore showed a stronger trend as an independent predictor. In contrast to studies of GIST, our study did not show any association between tumor size and outcome. We believe that this is best explained by the fact that the majority (three quarters) of EGISTs are large (>10 cm) when first detected. Small (and presumably early) EGISTs are rarely encountered because they seldom produce symptoms that lead to detection. Two of our four cases that were smaller than 5 cm were detected during workup for unrelated conditions. Were it possible to obtain a large enough group of EGIST of small size, we would speculate that such lesions would have an improved prognosis. There was also no correlation between the two histologic patterns noted in EGIST and outcome, although nonorganoid growth and a predominance of epithelioid areas have been associated with adverse outcome in the duodenum and jejunum/ileum, respectively. A recent study (13) of a small number of omental (nine cases) and mesenteric (seven cases) EGIST seemed to show a better prognosis for the former group. We are skeptical about accepting this statement at face value because of the extremely small sample size, short follow-up, and lack of stratification of these cases for other parameters that arguably affect outcome. Nonetheless, this observation deserves further study. Because immunohistochemical data were available for only a subset of these tumors and immunostains generally were performed in recent cases in which follow-up was unavailable, we could not analyze these parameters statistically. However, it has been repeatedly shown in many studies that there is little if any correlation between a given immunophenotypic profile and behavior.
Finally, our study validates the existence of stromal tumors that arise exclusively outside the gastrointestinal tract. They display a similar range in histologic appearance and immunophenotypic profile as GIST (24, 25, 26, 27, 28). As a group, they seem to resemble small intestinal stromal tumors more so than gastric ones. However, skeinoid fibers, a common marker of many small intestinal stromal tumors, was not encountered in any tumor in our group, although they were rarely noted in a previous study (13). Because precise localization of abdominal tumors (i.e., origin in omentum versus mesentery) was not possible in most cases, we cannot comment on site-specific histologic differences in EGIST as has been done with GIST. It is tempting to speculate, however, that omental EGIST may more closely resemble gastric stromal tumors whereas mesenteric tumors may mirror small bowel stromal tumors. In all cases examined, c-kit receptor was strongly expressed in most cells, whereas only a minority expressed muscle antigens. On the basis of c-kit receptor expression in GIST, it has been suggested that these tumors recapitulate the phenotype of the gastrointestinal pacemaker cell (interstitial cell of Cajal) (29). The recent observation that interstitial cells of Cajal do not express CD34, whereas the fibroblasts of Auerbach's plexus do (30), complicates the issue of differentiation in stromal tumors or at least suggests that stromal tumors may display hybrid features. Whether the expression of c-kit receptor in EGIST implies the presence of pacemaker cells outside the gastrointestinal tract or the ability of mesenchymal elements to recapitulate this phenotype aberrantly is uncertain. Nonetheless, it is clear this tyrosine kinase receptor represents a sensitive and reliable means of identifying both gastrointestinal and extragastrointestinal forms of the disease.
In summary, EGISTs are an aggressive group of stromal tumors. In this respect, they share more in common with GIST located in the distal gastrointestinal tract. Although mitotic activity (>2/50 HPF), cellularity, and necrosis all are important parameters for adverse outcome in a univariate analysis, further study is needed to elucidate their potential as independent risk factors. We had postulated that extremely small lesions (<5 cm) would also have an excellent prognosis as is seen in GIST. Such lesions are rare and are often detected incidentally. The small number of cases (4) that fell into that category in our study did not allow adequate evaluation of that point. Possibly a larger number of such cases with follow-up might allow for statistical validation. Finally, we have shown that with an increasing number of adverse histologic parameters, the risk for adverse outcome increases. If one stratifies our patient group into those whose tumors have no or one adverse histologic feature versus those who have two to three, the differences are striking. Only 5% of patients who had no or one feature experienced an adverse outcome compared with 92% of patients who had two to three features, with similarly convincing differences in incidence rates. We emphasize that these figures reflect a conservative estimate of risk assessment because the average follow-up period in our study was only 24 months.
References
Appelman HD . Smooth muscle tumors of the gastrointestinal tract: what we know that Stout didn't know. Am J Surg Pathol 1986; 10 (Suppl 1)83–99.
Lewin K, Appelman HD . Tumors of the stomach. In: Atlas of tumor pathology. Washington, DC:Armed Forces Institute of Pathology; 1997.
Evans HL . Smooth muscle tumors of the gastrointestinal tract: a study of 56 cases followed for a minimum of 10 years. Cancer 1985; 56: 2242–2250.
Stout AP . Bizarre smooth muscle tumors of the stomach. Cancer 1962; 15: 400–409.
Appelman HD, Helwig EB . Gastric epithelioid leiomyoma and leiomyosarcoma (leiomyoblastoma). Cancer 1976; 38: 708–728.
Weiss SW, Sobin LH . WHO histologic typing of soft tissue tumors. Berlin:Springer Verlag; 1994.
Enzinger FM, Weiss SW . Soft tissue tumors. 3rd ed.St. Louis:C.V. Mosby; 1995.
Mazur MT, Clark HB . Gastric stromal tumors: a reappraisal of histogenesis. Am J Surg Pathol 1983; 7: 507–519.
Emory TS, Sobin LH, Lukes L, Lee DH, O'Leary TJ . Prognosis of gastrointestinal smooth muscle (stromal) tumors. Am J Surg Pathol 1999; 23: 82–87.
Franquemont DW . Differentiation and risk assessment of gastrointestinal stromal tumors. Am J Clin Pathol 1995; 103: 41–47.
Brainard JA, Goldblum JR . Stromal tumors of the jejunum and ileum: a clinicopathologic study of 39 cases. Am J Surg Pathol 1997; 21: 407–416.
Goldblum JR, Appelman HD . Stromal tumors of the duodenum: a histologic and immunohistochemical study of 20 cases. Am J Surg Pathol 1995; 19: 71–80.
Miettinen M, Monihan JM, Sarlomo-Rikala M, Kovatich AJ, Carr NJ, Emory TS, et al. Gastrointestinal stromal tumors/smooth muscle tumors (GISTs) primary in the omentum and mesentery: clinicopathologic and immunohistochemical study of 26 cases. Am J Surg Pathol 1999; 23: 1109–1118.
Min K-W . Small intestinal stromal tumors with skeinoid fibers: clinicopathologic, immunohistochemical, and ultrastructural observations. Am J Surg Pathol 1992; 16: 145–155.
Newman PL, Wadden C, Fletcher CDM . Gastrointestinal stromal tumours: correlation of immunophenotype with clinicopathological features. Histopathology 1991; 164: 107–117.
Suster S . Gastrointestinal stromal tumors. Semin Diagn Pathol 1996; 13: 297–313.
Tworek JA, Appelman HD, Singleton TP, Greenson JK . Stromal tumors of the jejunum and ileum. Mod Pathol 1997; 10: 200–209.
Tworek JA, Goldblum JR, Weiss SW, Greenson JK, Appelman HD . Stromal tumors of the abdominal colon: a clinicopathologic study of 20 cases. Am J Surg Pathol 1999; 23: 937–945.
Tworek JA, Goldblum JA, Weiss SW, Greenson JK, Appelman HD . Stromal tumors of the anorectum: a clinicopathologic study of 22 cases. Am J Surg Pathol 1999; 23: 946–954.
Ueyama T, Guo K-J, Hashimoto H, Daimaru Y, Enjoji M . A clinicopathologic and immunohistochemical study of gastrointestinal stromal tumors. Cancer 1992; 69: 947–955.
Rosner B . Fundamentals of biostatistics. 4th ed.Belmont:Wadsworth; 1995.
Erlandson RA, Klimstra D, Woodruff JM . Subclassification of gastrointestinal stromal tumors based on evaluation by electron microscopy and immunohistochemistry. Ultrastruct Pathol 1996; 20: 373–393.
Pizzimbono CA, Higa E, Wise L . Leiomyoblastoma of the lesser sac: case report and review of the literature. Am Surg 1973; 39: 692–699.
Hurlimann J, Gardiol D . Gastrointestinal stromal tumours: an immunohistochemical study of 165 cases. Histopathology 1991; 19: 311–320.
Ma CK, Amin MB, Kintanar E, Linden MD, Zarbo RJ . Immunohistochemical characterization of gastrointestinal stromal tumors: a study of 82 cases compared with 11 cases of leiomyomas. Mod Pathol 1993; 6: 139–144.
Miettinen M, Virolainen M, Maarit, Sarlomo R . Gastrointestinal stromal tumors—value of CD34 antigen in their identification and separation from leiomyomas and schwannomas. Am J Surg Pathol 1995; 19: 207–216.
Monihan JM, Carr NJ, Sobin LH . CD 34 immunoexpression in stromal tumours of the gastrointestinal tract and in mesenteric fibromatoses. Histopathology 1994; 25: 469–473.
Van de Rijn M, Hendrickson MR, Rouse RV . CD34 expression by gastrointestinal tract stromal tumors. Hum Pathol 1994; 25: 766–771.
Kindblom LG, Remotti HE, Aldenborg F, Meis-Kindblom JM . Gastrointestinal pacemaker cell tumor (GIPACT): gastrointestinal stromal tumors show phenotypic characteristics of the interstitial cells of Cajal. Am J Pathol 1998; 152: 1259–1269.
Vanderwinden J-M, Rumessen JJ, DeLaet M-H, Vanderhaeghen J-J, Schiffmann SN . CD34+ cells in human intestine are fibroblasts adjacent to, but distinct from, interstitial cells of Cajal. Lab Invest 1999; 79: 59–65.
Author information
Authors and Affiliations
Corresponding author
Additional information
Presented in part at the United States and Canadian Academy of Pathology meeting, Washington, D.C., March 1996.
Rights and permissions
About this article
Cite this article
Reith, J., Goldblum, J., Lyles, R. et al. Extragastrointestinal (Soft Tissue) Stromal Tumors: An Analysis of 48 Cases with Emphasis on Histologic Predictors of Outcome. Mod Pathol 13, 577–585 (2000). https://doi.org/10.1038/modpathol.3880099
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3880099
Keywords
This article is cited by
-
An unusual presentation of an extragastrointestinal stromal tumour: a case report
Egyptian Journal of Radiology and Nuclear Medicine (2020)
-
Primary Hepatic Gastrointestinal Stromal Tumour (GIST): Unusual Presentation and Diagnosis
Journal of Gastrointestinal Cancer (2020)
-
Retroperitoneal cystic masses: magnetic resonance imaging features
Abdominal Radiology (2020)
-
A gastrointestinal stromal tumour mimicking solid pseudopapillary neoplasia of the pancreas—a case report
European Surgery (2018)
-
Extragastrointestinal stromal tumor of the inferior vena cava: a case report
Surgical Case Reports (2017)