Abstract
In anaplastic large-cell lymphomas positive for anaplastic lymphoma kinase (ALK) protein, the ALK gene is most commonly fused to the NPM gene, and less commonly to TPM3, TFG, ATIC, and other rare genes. Although this lymphoma is generally associated with a favorable clinical outcome, 25% of the patients die of the disease within 5 years. In this study, we developed three assays, all of which can be used with archival formalin-fixed, paraffin-embedded tissues: (1) a sensitive reverse transcription-polymerase chain reaction (RT-PCR) assay for various X-ALK fusion genes, (2) a 5′ rapid amplification of cDNA ends (RACE) assay to identify unknown fusion partners, and (3) a real-time RT-PCR assay to quantify the amount of the NPM-ALK fusion transcript. In 26 cases of ALK+ anaplastic large-cell lymphoma, the RT-PCR assay showed that the ALK was fused to NPM in 21 cases, to TPM3 in three, and to TFG in one. The 5′ RACE assay detected ATIC-ALK fusion in the remaining case. The real-time quantitative RT-PCR assay showed that the NPM-ALK transcript was over expressed in four of 20 quantifiable cases. Patients with NPM-ALK overexpression showed a significantly unfavorable overall survival compared with those with a low expression of this transcript. The RT-PCR and 5′ RACE assays developed here may be useful for identification of known and unknown gene partners fused to the ALK gene. Overexpression of the NPM-ALK fusion transcript may be associated with a poor prognosis of the patients with ALK+ anaplastic large-cell lymphomas.
Similar content being viewed by others
Introduction
Anaplastic large-cell lymphoma is a systemic T-cell non-Hodgkin's lymphoma.1 In more than half of these cases, tumor cells express anaplastic lymphoma kinase (ALK) protein, the expression of which is due to genetic alteration of the ALK gene locus on chromosome 2.2, 3 ALK+ anaplastic large-cell lymphomas are associated with a younger male patient. Although they often present at an advanced clinical stage, the response to chemotherapy is good, and the clinical outcome is favorable, as compared with ALK− anaplastic large-cell lymphomas.4, 5, 6 Nevertheless, one-fourth of patients still die of the disease within 5 years.
The commonest genetic alteration in ALK+ anaplastic large-cell lymphoma, found in 70–80% of cases, is a t(2;5)(p23;q35) translocation between the ALK gene on chromosome 2 and the nucleophosmin (NPM) gene on chromosome 5.7, 8 The ALK gene encodes a tyrosine kinase receptor belonging to the insulin growth factor receptor superfamily, which is normally expressed in nerve cells7, 9 and in human neuroblastoma cells,10 but is silent in normal lymphoid cells.9 It has been demonstrated that constitutive ALK activity contributes to the malignant transformation of lymphoid cells.11, 12, 13 In the remaining 20–30% of ALK+ cases, ALK gene is fused to various partners including TPM (tropomyocin) 3,14 TPM4,15 TFG (TRK fused gene),16, 17 ATIC (5-aminoimidazole-4-carboxamide ribonucleotide formyltransferase/IMP cyclohydrolase),18, 19 CLTC (clathrin heavy chain),20 MSN (moesin),21, 22 ALO17 (KIAA1618),23 and MYH (non-muscle myosin heavy chain) 9.24 The TPM3 gene has been the second most common fusion partner, and is involved in 10–20% of all ALK+ anaplastic large-cell lymphomas, while involvement of other genes seems to be rare.2, 3 The ALK fusion partners can be estimated from the ALK protein subcellular distribution pattern as detected by ALK immunohistochemistry.9, 25 In NPM-ALK cases, the ALK signal is detected in both cytoplasm and nucleus. This distribution may be explained by the functions of NPM that shuttles continuously between the cytoplasm to the nucleus.26 In contrast, in anaplastic large-cell lymphoma cases with fusions other than NPM-ALK, the ALK signal is detected in the cytoplasm, and rarely in the membrane.1, 21, 22 ALK immunohistochemistry, although easy to perform, has some shortcomings: non-NPM-ALK cases are sometimes misdiagnosed as NPM-ALK cases because of non-specific nuclear staining, and further classification of the former cases would be very difficult.
It has been reported that mouse fibroblast clones each stably transfected with respective ALK fusion genes showed distinct tumor properties,27 suggesting that both identification of the ALK fusion partners and the quantification of the fusion gene expression may have clinical implications. In this study, we have developed three assays: (1) a sensitive reverse transcription (RT)-polymerase chain reaction (PCR) assay for various X-ALK fusion genes, (2) a 5′ rapid amplification of cDNA ends (RACE) assay to identify unknown ALK fusion partners, and (3) a real-time RT-PCR assay to quantify the amount of the NPM-ALK fusion transcript. The advantages of being able to use routinely prepared paraffin materials far outweigh any demerits. All three assays presented here can be used with archival formalin-fixed, paraffin-embedded tissues. For 26 cases of ALK+ anaplastic large-cell lymphoma, we identified all ALK fusion partners using RT-PCR and 5′ RACE. In the NPM-ALK cases, the amount of the fusion transcript was quantified using real-time RT-PCR,28, 29 and the association between its overexpression and the clinicopathological characteristics was studied.
Materials and methods
Clinical Cases and Cell Line
Twenty-six cases of ALK+ anaplastic large-cell lymphoma were retrieved from the pathology files of Nagoya City University and other hospitals. Specimens were obtained at the initial presentation of the patients, fixed in formalin, and embedded in paraffin. This study was conducted according to the Declaration of Helsinki. All cases were reviewed carefully, and the histological diagnosis of anaplastic large-cell lymphoma was made according to criteria of the WHO classification of malignant lymphoma.1 All cases were nodal tumors. Immunohistochemically, CD30 was positive in 26/26 cases, CD3 in 10/26, CD4 in 5/16, CD20 in 0/26, CD43 in 14/20, epithelial membrane antigen in 21/21, and cytotoxic associated antigens (granzyme B or TIA-1) in 20/20. In situ hybridization for Epstein–Barr virus-encoded small RNA (EBER) was negative in 26/26 cases. All cases included in this study were positive for ALK as determined with anti-ALK monoclonal antibody (ALK-1, DAKO Cytomation, Kyoto, Japan). As shown in Table 1, ALK expression was detected in both nuclei and cytoplasm in 21 cases, and in the remaining five, it was restricted to the cytoplasm. One case (case 4) showed hemophagocytic syndrome, but had no particular history or underlying disorders that would predispose to such a syndrome. Cytogenetic data on the tumors were available in five cases, and translocations were as follows: t(2;5)(p23;q35) for NPM-ALK fusion in three cases, t(1;2)(q25;p23) for TPM3-ALK fusion in one case, and t(2;3)(p23;q35) for TFG-ALK fusion in the remaining case. These cases were used as positive controls for the RT-PCR assay for the respective fusion genes. The Karpas 299 cell line, which was established from an anaplastic large-cell lymphoma cell line carrying the NPM-ALK fusion gene, was used as a standard for quantification of the NPM-ALK fusion transcript, and normal lymph nodes were used as negative control.
Histology, MIB-1 Index, and Immunohistochemistry for CD56
Of the 26 anaplastic large-cell lymphoma cases, 20 were histologically classified as the common type, three cases as a lymphohistiocytic variant, and three cases as a small cell variant. To estimate proliferation activity, immunohistochemistry was performed with MIB-1 monoclonal antibody (DAKO Cytomation). The percentage of positive cells (MIB-1 index) was calculated by counting >500 tumor cells in the most positive areas. Expression of CD56 was detected using anti-CD56 monoclonal antibody (clone 1B6; Novocastra, Newcastle Upon Tyne, UK).
RNA Extraction
Total RNA was extracted from formalin-fixed, paraffin-embedded sections, as described previously.30 Briefly, deparaffinized sections were incubated at 56°C overnight in proteinase K digestion buffer. Total RNA was extracted with concentrated phenol/guanidine isothiocyanate (Trizol LS, Invitrogen, Carlsbad, CA, USA), then subjected to RNase-free DNase I treatment, and finally re-suspended in RNase-free water. Total RNA was similarly extracted from Karpas 299 tumor cells.
RT-PCR Detection of Fusion Transcript
The fusion transcripts (NPM-ALK, TPM3-ALK, and TFG-ALK) were detected using a two-step RT-PCR. Briefly, cDNA was synthesized from the total RNA using random hexamers (SuperScript First-Strand Synthesis System kit, Invitrogen, Carlsbad, CA, USA). Forty-five cycles of PCR was performed with ALK-3 primer and the respective primers (NPM-1, TPM3-1, and TFG-1) as shown in Table 2. After amplification, PCR products were electrophoresed in 3% agarose gel, and directly sequenced by means of cycle sequencing with dye-labeled terminators (BigDye Terminator Cycle Sequencing kit, Applied Biosytems, Foster City, CA, USA) and analyzed on an automated DNA sequencer (Model 310, Applied Biosytems). Positive controls for NPM-ALK, TPM3-ALK, and TFG-ALK fusion transcripts consisted of anaplastic large-cell lymphoma cases possessing t(2;5), t(1;2), and t(2;3), respectively. The RNA quality was confirmed by RT-PCR amplification of the β-actin transcript using the primers, Actin-1 and Actin-2 (Table 2).
5′ Rapid Amplification of cDNA Ends
Identification of unknown ALK partner genes was performed with a 5′ RACE technique (SMARTTM RACE cDNA Amplification kit, Clontech, Mountain View, CA), using total RNA extracted from formalin-fixed, paraffin-embedded tissues. The first-strand 5′ RACE cDNA was synthesized using a gene-specific primer (ALK-1 and SMART IIA, Table 2). The first-round PCR was performed with the primers, ALK-2 primer and the Nested Universal Primer (NUP), followed by seminested PCR, using the primers, ALK-3 and NUP. The final products of 100–200 bp were cloned into pGEM-T easy vector (Promega, Madison, WI, USA), and directly sequenced. Sequence homologies were identified using the BLAST program of the National Center for Biotechnology Information available at http://www.ncbi.nlm.nih.gov/BLAST/.
Real-Time Quantitative RT-PCR for the NPM-ALK Fusion Transcript
Real-time quantitative RT-PCR analysis for NPM-ALK and β-actin mRNA (an endogenous reference) was carried out with the ABI PRISM 7300 Real-Time PCR System (Applied Biosystems) using SYBR Green PCR Master Mix (Applied Biosystems). cDNA synthesized with random hexamers was employed. The real-time PCR was performed in a 20 μl final reaction mixture that included diluted cDNA and 0.5 pmol/μl of each primer (NPM-1 and ALK-3 for NPM-ALK and Actin-1 and Actin-2 for β-actin, Table 2). Amplification conditions were 2 min at 50°C and 10 min at 95°C, followed by 45 cycles of 15 s at 95°C and 1 min at 60°C, and finally by a dissociation stage (15 s at 95°C, 30 s at 60°C, and 15 s at 95°C).
For construction of the standard curves, 10-fold serial dilutions of cDNA of Karpas 299 were prepared for both target NPM-ALK and β-actin. A preliminary study showed that the coefficient of determination (R2) and the slope value for NPM-ALK were 0.999 and −3.39, respectively, and those for β-actin were 0.995 and −3.17, respectively. A standard curve was created with each run, and the threshold cycle was used for quantification of the input target number. The amount of NPM-ALK was divided by that of β-actin. This value was further divided by the tumor cell ratio in the section, which was counted under a microscope using H&E and ALK-immunostained sections as a guide, then a normalized target value was finally obtained. The real-time PCR was performed in triplicate for each case, and the results were averaged. Melting curve analysis was also performed after PCR amplification to confirm that there were no primer dimers in the PCR products.
Statistical Analysis
Statistical evaluation of data from two groups was performed using Fischer's exact test and Student's t-test. All analyses were two-tailed. To identify the parameters significantly associated with an overall survival, the survival rate was calculated by the Kaplan–Meier method, and the statistical difference was estimated using the log-rank and Wilcoxon tests. A value of P<0.05 for each test was regarded as statistically significant. All of the analyses were performed using the statistical package JMP, version 5 (SAS Institute Inc., Cary, NC, USA).
Results
RT-PCR Assay for ALK Fusion Partners
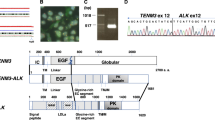
To determine the genes fused to ALK, we performed an RT-PCR assay with primer pairs specific to respective fusion types using RNA extracted from paraffin sections (Figure 1). Among the 26 cases that were positive on ALK immunohistochemistry, the NPM-ALK fusion transcript (92 bp) was detected in 21, TPM3-ALK (105 bp) in three, and TFG-ALK (120 bp) in one. In the remaining case, the ALK fusion partner was not determined (case 26). All cases positive for the NPM-ALK fusion transcript showed a nuclear and cytoplasmic ALK staining pattern (Supplementary Information 1A, 1B). Three cases with the TPM3-ALK fusion, one case with the TFG-ALK fusion (Supplementary Information 1C), and one partner-unknown case (case 26) showed a cytoplasmic ALK staining pattern.
An RT-PCR assay for NPM-ALK, TPM3-ALK, TFG-ALK, and ATIC-ALK fusion transcripts. (a) NPM-ALK (92 bp); (b) TPM3-ALK (105 bp); (c) TFG-ALK (120 bp); and (d) ATIC-ALK (102 bp). N, negative control (normal lymph node); lanes 1–10, ALK+ anaplastic large-cell lymphomas. β-actin mRNA is amplified in all cases.
5′ RACE Assay
To identify the possible fusion partner in the partner-unknown case (Case 26) by RT-PCR, we performed a 5′ RACE assay using total RNA extracted from the paraffin-embedded tissue. Direct sequencing showed that five of six clones obtained from this assay possessed an identical sequence. Comparing of this sequence with those obtained by nucleotide database searches revealed that exon 7 of the ATIC gene was fused to exon 20 of the ALK gene. To confirm the presence of the ATIC-ALK fusion transcript in this case, cDNA synthesized using random hexamers was subjected to PCR using primers, ALK-3 and ATIC-1 (Table 2). The direct sequencing of the RT-PCR product (102 bp) showed that the ATIC-ALK fusion transcript (Figure 1, lane 10) had identical break points.
Clinicopathological Features of ALK+ Anaplastic Large-Cell Lymphomas
As shown in Table 1, there were 13 males and 13 females with ages ranging from 1 to 46 years (mean, 15 years). MIB-1 index, calculated in 25 cases, ranged from 11 to 92% with a mean of 65%. Three cases were immunohistochemically positive for CD56 (Cases 5, 9, and 13) as shown in Supplementary Information 1D. The clinical data were available from 25 patients. Six cases had a less advanced clinical stage (one case at stage I and five at stage II) and 19 cases had an advanced clinical stage (13 cases at stage III and six at stage IV). Thirteen patients exhibited systemic symptoms (B-symptoms) such as night sweats, fever, and weight loss. One patient presented with hemophagocytic syndrome (Case 4). All cases were treated with anthracyclin-based combination chemotherapy. One case underwent autologous peripheral blood stem cell transplantation after induction of complete remission (Case 11). Follow-up data were available in 24 patients. The follow-up periods ranged from 3 to 144 months (mean 34 months). Three of 20 patients with NPM-ALK tumors died of the disease while those with non-NPM-ALK tumors were alive at the last follow-up. Clinicopathological features and overall survival were not statistically different between patients with NPM-ALK and non-NPM-ALK tumors.
Real-Time Quantitative RT-PCR for NPM-ALK Fusion Transcript
To test the reproducibility of the real-time quantitative RT-PCR using RNA extracted from paraffin sections, we determined normalized target amounts of the transcript in the Karpas 299 cell line and in two clinical cases with the NPM-ALK fusion. Reproducible results were obtained in each case, and amounts in the two clinical cases were identical at the corresponding dilutions. These data indicated that the NPM-ALK fusion transcript could be reliably quantified in our real-time RT-PCR assay. Among the 21 cases with this transcript, 20 cases were quantifiable (Table 1). The normalized NPM-ALK values ranged from 0.5 to 31.7 (mean 6.7). We created a scatter diagram and divided our cases into two groups (Figure 2): high NPM-ALK expressers (cases 2, 4, 6, and 12) and low expressers (other cases). Normal lymph nodes showed no detectable NPM-ALK fusion transcript. The NPM-ALK expression level was not significantly associated with ALK expression or other immunophenotypes of the tumor cells.
Impact of High Expression of the NPM-ALK Fusion Transcript on Clinicopathological Characteristics
Twenty cases quantifiable of the NPM-ALK fusion transcript (Cases 1–20) consisted of nine males and 11 females with a mean age of 12.8 years. None of the seven clinicopathological factors analyzed (age, sex, clinical stage, B-symptoms, histological type, MIB-1 index, and CD56 expression) showed any significant association with the NPM-ALK expression level. A survival analysis showed that high NPM-ALK expressers presented with significantly reduced overall survivals than did the low expressers with respective 5-year survival rates of 25 and 100% (Figure 3, log-rank P=0.0064, Wilcoxon P=0.0024). Other factors had no significant impact on survival.
Discussion
Among the subgroups of anaplastic large-cell lymphoma, ALK expression defines a distinct form, which is associated with a young age and a favorable prognosis compared with ALK− anaplastic large-cell lymphoma.4, 5, 6 However, approximately 25% of patients with ALK+ anaplastic large-cell lymphoma still die of the disease within 5 years, thus highlighting the need to find risk factors that identify potential patients who would be better served by risk-adjusted therapies.4, 5, 6 Several risk factors have been reported for ALK+ anaplastic large-cell lymphoma: the International Prognostic Index,6, 31 survivin expression,32 and CD56 expression.31 Recently, Armstrong et al27 reported the transforming potential of X-ALK fusion proteins using mouse fibroblast clones stably transfected with five different X-ALK cDNAs (NPM-ALK, TPM3-ALK, TFG-ALK, CLTC-ALK and ATIC-ALK). Each clone showed distinct cell properties. The proliferation rate was associated with the level of X-ALK expression except for TPM3-ALK. The highest invasion capacity was shown by the TPM3-ALK clone, and the highest tumorigenicity was observed in the NPM-ALK and TFG-ALK clones. These observations suggest that identification and quantification of ALK fusion partners may have clinical implications in the pathogenesis and prognosis of ALK+ anaplastic large-cell lymphomas.
The most important finding of this study is that in anaplastic large-cell lymphomas with the NPM-ALK fusion, the high expression of the fusion transcript was associated with an unfavorable overall survival. We successfully quantified the NPM-ALK fusion transcript using RNA extracted from paraffin sections. It is worth noting that NPM-ALK expression levels were not associated with any of the clinicopathological factors analyzed such as age, sex, clinical stage, B-symptoms, histological type, MIB-1 index, and CD56 expression. These findings suggest that NPM-ALK overexpression may be a novel risk factor independent of known risk factors such as the clinical stage and CD56 expression.6, 31 The direct link between overexpression of the transcript and an unfavorable prognosis is currently unknown. Constitutively active NPM-ALK fusion protein, thus far evaluated only in vitro, may activate a number of downstream effectors, including phospholipase C-γ, RAS, signal transducer and activator of transcription proteins, and phosphoinositol 3′-kinase, and may be associated with both cell growth and apoptosis regulation.33 In this study, no association was noted between NPM-ALK overexpression and tumor cell proliferation (MIB-1 index). Unlike malignant epithelial tumors, cell proliferation is not generally considered as a risk factor in malignant lymphoma. Patients with Burkitt's lymphoma, which is characterized by a high tumor cell proliferation, have a favorable prognosis with the treatment of very intensive chemotherapy.34 Presumably, NPM-ALK overexpression may affect other tumor characteristics such as the invasion capacity, tumorigenicity, and antiapoptosis through differential activation of various signaling pathways.27, 33
The second important achievement of this study was the successful identification of ALK-fusion partners by RT-PCR using paraffin sections as a source of RNA. Although ALK immunohistochemistry provides a useful means for identifying these partners,9, 25 misinterpretation of the staining pattern (nuclear and cytoplasmic vs cytoplasmic) can sometimes occur, and subclassification of non-NPM-ALK tumors is difficult in most cases. Several RT-PCR assays for X-ALK fusion transcripts have been reported.3, 7, 25 In our preliminary assays, we used RNA extracted from paraffin materials to these assays, but found that sensitivities were not satisfactory (unpublished data). Results may be improved by optimizing RT-PCR assays for use with such degraded RNA, and the RT-PCR assay developed here showed a high degree of sensitivity and specificity in the detection of ALK fusion partners. Simple to perform, this assay is expected to become a powerful tool for screening of non-NPM-ALK cases. At present, statistical analyses have been incapable of distinguishing prognostically between NPM-ALK cases and non-NPM-ALK cases. However, the latter cases might have a better overall survival as estimated from our data and those of Falini et al.35 It was also found that non-NPM-ALK translocations are common in pediatric anaplastic large-cell lymphomas.36
Another important finding of this study is that we identified an ALK fusion partner by a 5′ RACE technique using paraffin sections as a source of RNA. To the best of our knowledge, this is the first report of a 5′ RACE assay that has been successfully performed using paraffin sections. To date, nine different ALK partner genes have been reported, and there are likely more to be identified. In determining these partners, the RNA has to be of high quality. Our 5′ RACE technique described here will be useful for identification of unknown ALK fusion partners when only routinely prepared histological materials are available. Our present success with 5′ RACE may be due to the following: one is that the break point of the ALK gene has been restricted to a narrow portion around the junction between exons 19 and 20, and (2) the wild-type ALK transcript is virtually absent in anaplastic large-cell lymphoma tissue, which helps increase 5′ RACE sensitivity. Identification of novel fusion partners is important in clarifying the pathogenesis of ALK+ anaplastic large-cell lymphoma. For example, the recently identified MYH9-ALK fusion lacks a functional oligomerization domain,24 which has been described as critical for the anaplastic large-cell lymphoma tumorigenesis.37
Among ALK+ anaplastic large-cell lymphomas, ALK most commonly fuses with NPM, constituting more than 70% of the total number of cases. We showed that overexpression of the NPM-ALK fusion transcript was associated with a poor overall survival. To establish the prognostic significance of this parameter, a large-scale study is needed. Moreover, the accurate selection of NPM-ALK cases is essential. The RT-PCR assay presented here would be useful for this selection. Furthermore, it is expected to be a powerful tool in the collection of non-NPM-ALK anaplastic large-cell lymphomas whose precise clinicopathological characteristics remain to be clarified. Lastly, the 5′ RACE technique described here should prove to be useful in identifying novel genes that fuse with the ALK gene in anaplastic large-cell lymphomas. It should be emphasized that routinely processed paraffin materials can be used in all these assays, which will greatly facilitate future anaplastic large-cell lymphoma studies.
References
Delsol G, Ralfkiaer E, Stein H, et al. Anaplastic large cell lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW (eds). World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press: Lyon, 2001, pp 230–235.
Stein H, Foss HD, Durkop H, et al. CD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood 2000;96:3681–3695.
Drexler HG, Gignac SM, von Wasielewski R, et al. Pathobiology of NPM-ALK and variant fusion genes in anaplastic large cell lymphoma and other lymphomas. Leukemia 2000;14:1533–1559.
Shiota M, Nakamura S, Ichinohasama R, et al. Anaplastic large cell lymphomas expressing the novel chimeric protein p80NPM/ALK: a distinct clinicopathologic entity. Blood 1995;86:1954–1960.
Falini B, Pileri S, Zinzani PL, et al. ALK+ lymphoma: clinico-pathological findings and outcome. Blood 1999;93:2697–2706.
Gascoyne RD, Aoun P, Wu D, et al. Prognostic significance of anaplastic lymphoma kinase (ALK) protein expression in adults with anaplastic large cell lymphoma. Blood 1999;93:3913–3921.
Morris SW, Kirstein MN, Valentine MB, et al. Fusion of a kinase gene, ALK, to a nucleolar protein gene, NPM, in non-Hodgkin's lymphoma. Science 1994;263:1281–1284.
Fujimoto J, Shiota M, Iwahara T, et al. Characterization of the transforming activity of p80, a hyperphosphorylated protein in a Ki-1 lymphoma cell line with chromosomal translocation t(2;5). Proc Natl Acad Sci USA 1996;93:4181–4186.
Pulford K, Lamant L, Morris SW, et al. Detection of anaplastic lymphoma kinase (ALK) and nucleolar protein nucleophosmin (NPM)-ALK proteins in normal and neoplastic cells with the monoclonal antibody ALK1. Blood 1997;89:1394–1404.
Lamant L, Pulford K, Bischof D, et al. Expression of the ALK tyrosine kinase gene in neuroblastoma. Am J Pathol 2000;156:1711–1721.
Kuefer MU, Look AT, Pulford K, et al. Retrovirus-mediated gene transfer of NPM-ALK causes lymphoid malignancy in mice. Blood 1997;90:2901–2910.
Chiarle R, Gong JZ, Guasparri I, et al. NPM-ALK transgenic mice spontaneously develop T-cell lymphomas and plasma cell tumors. Blood 2003;101:1919–1927.
Miething C, Grundler R, Fend F, et al. The oncogenic fusion protein nucleophosmin-anaplastic lymphoma kinase (NPM-ALK) induces two distinct malignant phenotypes in a murine retroviral transplantation model. Oncogene 2003;22:4642–4647.
Lamant L, Dastugue N, Pulford K, et al. A new fusion gene, TPM3-ALK, in anaplastic large-cell lymphoma created by a (1;2) (q25;p23) translocation. Blood 1999;93:3088–3095.
Lawrence B, Perez-Atayde A, Hibbard MK, et al. TPM3-ALK and TPM4-ALK oncogenes in inflammatory myofibroblastic tumors. Am J Pathol 2000;157:377–384.
Hernandez L, Pinyol M, Hernandez S, et al. TRK-fused gene (TFG) is a new partner of ALK in anaplastic large cell lymphoma producing two structurally different TFG-ALK translocations. Blood 1999;94:3265–3268.
Hernandez L, Bea S, Bellosillo B, et al. Diversity of genomic breakpoints in TFG-ALK translocations in anaplastic large cell lymphomas: identification of a new TFG-ALK(XL) chimeric gene with transforming activity. Am J Pathol 2002;160:1487–1494.
Trinei M, Lanfrancone L, Campo E, et al. A new variant anaplastic lymphoma kinase (ALK)-fusion protein (ATIC-ALK) in a case of ALK-positive anaplastic large cell lymphoma. Cancer Res 2000;60:793–798.
Colleoni GW, Bridge JA, Garicochea B, et al. ATIC-ALK: a novel variant ALK gene fusion in anaplastic large cell lymphoma resulting from the recurrent cryptic chromosomal inversion, inv(2)(p23q35). Am J Pathol 2000;156:781–789.
Touriol C, Greenland C, Lamant L, et al. Further demonstration of the diversity of chromosomal changes involving 2p23 in ALK-positive lymphoma: 2 cases expressing ALK kinase fused to CLTCL (clathrin chain polypeptide-like). Blood 2000;95:3204–3207.
Tort F, Pinyol M, Pulford K, et al. Molecular characterization of a new ALK translocation involving moesin (MSN-ALK) in anaplastic large cell lymphoma. Lab Invest 2001;81:419–426.
Tort F, Campo E, Pohlman B, et al. Heterogeneity of genomic breakpoints in MSN-ALK translocations in anaplastic large cell lymphoma. Hum Pathol 2004;35:1038–1041.
Cools J, Wlodarska I, Somers R, et al. Identification of novel fusion partners of ALK, the anaplastic lymphoma kinase, in anaplastic large-cell lymphoma and inflammatory myofibroblastic tumor. Genes Chromosomes Cancer 2002;34:354–362.
Lamant L, Gascoyne RD, Duplantier MM, et al. Non-muscle myosin heavy chain (MYH9): a new partner fused to ALK in anaplastic large cell lymphoma. Genes Chromosomes Cancer 2003;37:427–432.
Shiota M, Fujimoto J, Takenaga M, et al. Diagnosis of t(2;5)(p23;q35)-associated Ki-1 lymphoma with immunohistochemistry. Blood 1994;84:3648–3652.
Bischof D, Pulford K, Mason DY, et al. Role of the nucleophosmin (NPM) portion of the non-Hodgkin's lymphoma-associated NPM-anaplastic lymphoma kinase fusion protein in oncogenesis. Mol Cell Biol 1997;17:2312–2325.
Armstrong F, Duplantier MM, Trempat P, et al. Differential effects of X-ALK fusion proteins on proliferation, transformation, and invasion properties of NIH3T3 cells. Oncogene 2004;23:6071–6082.
Specht K, Kremer M, Muller U, et al. Identification of cyclin D1 mRNA overexpression in B-cell neoplasias by real-time reverse transcription-PCR of microdissected paraffin sections. Clin Cancer Res 2002;8:2902–2911.
Cronin M, Pho M, Dutta D, et al. Measurement of gene expression in archival paraffin-embedded tissues: development and performance of a 92-gene reverse transcriptase-polymerase chain reaction assay. Am J Pathol 2004;164:35–42.
Inagaki H, Okabe M, Seto M, et al. API2-MALT1 fusion transcripts involved in mucosa-associated lymphoid tissue lymphoma: multiplex RT-PCR detection using formalin-fixed paraffin-embedded specimens. Am J Pathol 2001;158:699–706.
Suzuki R, Kagami Y, Takeuchi K, et al. Prognostic significance of CD56 expression for ALK-positive and ALK-negative anaplastic large-cell lymphoma of T/null cell phenotype. Blood 2000;96:2993–3000.
Schlette EJ, Medeiros LJ, Goy A, et al. Survivin expression predicts poorer prognosis in anaplastic large-cell lymphoma. J Clin Oncol 2004;22:1682–1688.
Kutok JL, Aster JC . Molecular biology of anaplastic lymphoma kinase-positive anaplastic large-cell lymphoma. J Clin Oncol 2002;20:3691–3702.
Diebold J, Jaffe ES, Raphael M, et al. Burkitt lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW (eds). World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of Haematopoietic and Lymphoid Tissues. IARC Press: Lyon, 2001, pp 181–184.
Falini B, Pulford K, Pucciarini A, et al. Lymphomas expressing ALK fusion protein(s) other than NPM-ALK. Blood 1999;94:3509–3515.
Liang X, Meech SJ, Odom LF, et al. Assessment of t(2;5)(p23;q35) translocation and variants in pediatric ALK+ anaplastic large cell lymphoma. Am J Clin Pathol 2004;121:496–506.
Morris SW, Xue L, Ma Z, et al. Alk+ CD30+ lymphomas: a distinct molecular genetic subtype of non-Hodgkin's lymphoma. Br J Haematol 2001;113:275–295.
Acknowledgements
This work was supported in part by a Grant-in-Aid from the Ministry of Education, Science, Sports, and Culture of Japan to HI and CL. A Postdoctoral Fellowship for Foreign Researchers to CL from the Japan Society for the Promotion of Science (JSPS) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
Supplementary Information accompanies the paper on Modern Pathology website (http://www.nature.com/modpathol)
Supplementary information
Rights and permissions
About this article
Cite this article
Li, C., Takino, H., Eimoto, T. et al. Prognostic significance of NPM-ALK fusion transcript overexpression in ALK-positive anaplastic large-cell lymphoma. Mod Pathol 20, 648–655 (2007). https://doi.org/10.1038/modpathol.3800781
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800781