Abstract
Aberrant expression of human leukocyte antigen G (HLA-G) has been proposed to be involved in tumor escape mechanisms. It has been also proposed that detection of HLA-G might service as a potential biomarker for diagnosis or prediction of the clinical outcomes in ovarian and breast cancers, carcinoma of the lung and endometrial cancer. The aim of this current study is to determine if HLA-G is expressed in colorectal carcinomas and if the expression is associated with clinicopathological and prognostic data. The expression of HLA-G was investigated immunohistochemically in 201 patients with colorectal carcinomas. The correlation between HLA-G status, clinicopathological factors and the overall survival rate was analyzed. In this prospectively study, HLA-G protein expression was observed in 64.6% (130/201) of the primary site colorectal carcinomas, but not in the normal colorectal tissues or benign adenomas. HLA-G expression in the tumors was significantly correlated with the depth of invasion, histological grade, host immune response, lymph nodal metastasis and clinical stages of the disease (P=0.001, 0.0001, 0.002, 0.001 and 0.031, respectively). Patients with HLA-G positive tumors had a significantly shorter survival time than those patients with tumors that were HLA-G negative (P=0.0001). As well, in multivariate analysis, HLA-G demonstrated an independent prognostic factor (P=0.021, relative risk 3.14; 95% confidence interval, 1.34–8.10). Therefore, it can be gathered that HLA-G might serve as an independent prognostic factor for colorectal cancer patients.
Similar content being viewed by others
Main
Colorectal cancer is one of the most common cancers in the western world, and remains a major cause of cancer-related death in both men and women.1 Although colorectal cancer is less common in China, other than in the more economically developed regions, the incidences of colorectal cancer has been steadily increasing over the past few decades.2, 3
An assessment of prognosis, based on features of the resected tumor is valuable to the triage of patients who may benefit from adjuvant therapy. Currently, clinicopathological staging is the most important prognostic factor for determining the clinical outcome of colorectal cancer.4 However, patients with similar stages of the disease may have various outcomes. Therefore, there exists a great need to identify useful prognostic markers in guiding treatment decisions and/or in developing more effective treatments.
Human leukocyte antigen G (HLA-G) is a non-classical major histocompatibility (MHC) class Ib antigen. Its expression in normal tissues is limited to trophoblastic cells,5 placental chorionic endothelium,6 activated monocytes7 and thymic epithelial cells.8 However, it is not found in other healthy tissues expressing MHC class Ia antigens. In the neoplasm, HLA-G is expressed in all trophoblastic tumors,9 and in clinical specimens such as melanoma,10 renal cell carcinoma,11 carcinoma of the lung,12 breast carcinoma,13 lymphomas,14 ovarian carcinoma,15 endometrial adenocarcinoma16 and various gastrointestinal cancers including pancreatic ductal adenocarcinoma, ampullary cancer, biliary cancer and colorectal cancer.17
HLA-G has been postulated to mediate immunal tolerance during pregnancy by suppressing alloreactive CD4+ T-cell proliferation18, 19 and inhibiting NK-cell- and T-cell-mediated cytolysis.20, 21, 22 HLA-G expression in cancer cells has also been hypothesized to play a role in the evasion of immunosurveillance by host T-lymphocytes and NK cells.23 Interestingly, the detection of HLA-G was reported to be correlated with some clinicopathological parameters in lymphoma,24, 25, 26 melanoma,24, 27 ovarian and breast cancers,15, 28 carcinoma of the lung,12 and endometrial cancer.16 These studies have indicated that HLA-G might serve as a clinical marker for the diagnosis or prediction of the clinical outcomes of those diseases. The purpose of our study is to examine the histological sections of colorectal cancer for the presence of HLA-G protein, from which we can test our hypothesis that the immunohistochemical detection of HLA-G can be a reliable prognostic marker for patients with colorectal cancer.
Materials and methods
Patients and Tissue Samples
A total of 201 patients with colorectal adenocarcinoma, diagnosed and treated between 2002 and 2005 in three hospitals located in Chengdu, Sichuan, PR China, were investigated in our study. Of the 201 tumors evaluated, 40% (80/201) were colon in origin and 121 were primary rectal cancers. The mean age at the time of diagnosis was 64 years (s.d.=13.3 years), and there were 106 male patients and 95 female patients.
The patients had no previous diagnosis of carcinoma, and none of them had a history of hereditary colon cancer syndrome. In addition, none of them had preoperative chemotherapy or radiotherapy. However, after surgery, for both colon and rectal cancers, patients with stage I to III tumors received oral 5-fluorouracil and patients with stage IV tumors had 5-fluorouracil-based systemic chemotherapy without any radiation.
The clinicopathological findings were determined according to the classification of malignant tumors as set out by the International Union against tumor-node-metastasis (TNM).29 Out of the 201 cancer patients, 21% (42/201) were classified as being in stage I, 38% (77/201) were in stage II, 39% (78/201) were in stage III and 2% (4/201) were relegated to stage IV. Eighty-five available patients were followed up after the evaluation of HLA-G expression for a period of 36 months or until death. The average follow-up time for surviving patients was 27 months (range, 3–36 months) and during the follow-up period, there were 20 cancer-related deaths (23%).
All histological samples were taken from the primary lesion in the colon–rectum. No specimens from metastatic disease sites were included in this study. All tissue specimens underwent microscopic confirmation for pathological features before the inclusion in this study. Twenty sections of normal colorectal tissue and 20 adenomas were also examined. This study was approved and monitored by the ethics committee at each of the three hospitals.
Anti-HLA-G Monoclonal Antibody
A monoclonal (mAb) antibody against HLA-G named HGY was made by standard procedures from splenocytes of Balb/c mice that were immunized with HLA-G proteins purified from the first trimester placenta tissue.30, 31 Specificity for the HLA-G proteins was demonstrated by Western blotting and immunohistochemistry in the following manner. First, the mAb was able to detect both the surface (38–39 kDa) and secreted forms (34–35 kDa) of HLA-G proteins in cytotrophoblast and JEG-3 choriocarcinoma cell line cell lysate. Second, no classic class I molecules could be detected in several human cells expressing distinct HLA class I alleles and pooled white blood cell lysates. Third, the HLA-G proteins were able to stain to paraffin-embedded trophoblast tissue sections, but not to paraffin-embedded normal tissue sections.
Immunohistochemistry
Four-micrometer thick sections of the paraffin-embedded tissue blocks were cut and mounted on poly-lysine coated slides. They were dewaxed in xylene and rehydrated through a graded series of ethanol. After deparaffinization, antigen retrieval treatment was performed at 120°C (autoclave) for 5 min in a 10 nmol/l sodium citrate buffer (pH 6.0). Endogenous peroxidase activity was blocked by using a 0.03% hydrogen peroxide solution containing sodium azide at room temperature for 30 min. Next, anti-HLA-G monoclonal antibody (1:400) was incubated overnight at 4°C. After which, a thorough washing in a 0.01 M phosphate-buffered saline (PBS) solution was performed. Subsequently, binding sites of the primary antibody were visualized using a Dako EnVison kit (Dako, Denmark A/S) in accordance with the manufacturer's instructions. Finally, sections were faintly counterstained with hematoxylin and mounted with glycerol gelatin. Cytotrophoblast from first-trimester human placenta served as a positive protein control and negative controls were achieved by omissions of the primary antibody. The specificity of the HLA-G staining was further confirmed by a pre-incubation of the anti-HLA-G monoclonal antibody with blocking purified HLA-G protein. This in turn abolished cytotrophoblast staining.
Evaluation of Staining
All sections were blindly analyzed by two experienced pathologists (HY, KL or DD T) under a light microscope. Based on the estimated percentages of positive cells and/or the immunostaining intensity, which was determined by comparing the immunoreactivity of three positive controls that were included in each experiment, staining results were divided into four categories: (−) tissue specimens without staining, (+) tissue specimens with less than 25% of the cancer tissues and/or weakly stained, (++) tissue specimens with 25–50% of the cancer tissue and/or moderately stained and (+++) tissue specimens with more than 50% of the cancer tissue and/or strongly stained.
Host Immune Response Estimation
Host immune response was assessed by estimating the number of intratumoral lymphocyte infiltration in 76 colorectal cancer patients.32 Out of these patients, rectal and colon cancers were 47 (60%) and 29 (40%), respectively, and HLA-G positive and negative expressions were 67 and 33%, respectively. The proportions of colon vs rectal cancers as well as HLA-G positive vs negative expressions in the 76 cases studied were similar to that of the whole population. Based upon the estimated number of infiltrated lymphocytes within the areas of five high-power fields, host immune response features were scored as strong, moderate or weak.
Statistical Analysis
All statistical analyses were carried out with the SPSS software program. Correlations between the degree of staining and the subgroups according to the clinical and pathological classifications were calculated using the Pearson χ2 test. The Kaplan–Meier method was used to estimate the overall survival rate as a function of time. Survival differences were analyzed using the log-rank test. The Cox proportional hazard model was used for univariate and multivariate analysis of the prognostic factors. P<0.05 was considered to be significant.
Results
HLA-G Expression in Primary Colorectal Cancer
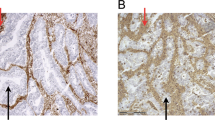
The immunohistochemical staining resulted in a visualization of the HLA-G as a brown-stained product. HLA-G was not stained in normal colorectal tissues (Figure 1a) and benign adenomas (Figure 1b). However, we observed HLA-G protein immunoreactivity in 65% (130/201) of malignant specimens, in which 18% (24/130) was regarded as strongly expressed (+++), 31% (41/130) was moderately expressed (++) and 50% (65/130) was weakly expressed (+). HLA-G was presented as either a membrane-associated (8%) or cytoplasmic pattern (2%). However, in the majority of cases (90%), it was presented as both (Figure 1c). Seventy-one cases of colorectal cancers (35%) were found to have negatively stained HLA-G.
Correlation with Clinicopathological Parameters
To seek the role of HLA-G expression in colorectal cancer, we next examined the correlation of HLA-G expression with the clinicopathological features such as age, gender, tumor locations, tumor types, depth of wall invasion, histological grade, host immune response, nodal status and stages of the disease (Table 1). We found a positive correlation between histological grades, depth of wall invasion and advanced disease stages (P=0.0001, 0.001 and 0.031, respectively). We also found a reverse correlation between HLA-G expression and host immune response (P=−0.002). As further illustrated in Figure 2, host immune response was weak in patients with a strong HLA-G expression whereas HLA-G negative patients were associated with a strong immune response. HLA-G expression was more frequent in colon cancers or in tumors located in the ascending and transverse colon (hence referenced as ‘proximal’) than in rectal cancers (P=0.017) or in the descending colon, sigmoid colon and rectum (hence referenced as ‘distal’) (P<0.0001). As shown in Table 2, further analysis found that there were more cases of the early cancer stage (stage I) in rectal cancers (26%) or distal tumors (27%) than that in colon cancer (12%) or proximal tumors (3%). This indicated that the differences between colon/proximal tumors and rectal/distal tumors were caused by the different cases of the disease stages in the studied population. In the case of nodal status, HLA-G expression frequency seemed to be higher in patients with nodal metastasis than those with negative nodes. However, this did not produce any statistical significance (P=0.07). Similarly, HLA-G expression in patients with nodal metastasis was significantly higher in colon/proximal tumors than in rectal/distal tumors (P=0.001) when the patients were further divided into colon or proximal and rectal or distal subgroups (Table 3). There was no significant correlation of HLA-G expression with respect to age or gender (P=0.620 and 0.876, respectively).
Correlation with Patients’ Survival
Within a period of 36 months of the post-assay follow-up, 20 cancer-related deaths occurred. Two of the deaths came from the 48 patients with HLA-G negative tumors, and 18 of the deaths were from the 37 patients in the HLA-G positive group. In the entire cohort, the overall survival rate of patients with HLA-G negative tumors were significantly higher when compared to those with HLA-G positive tumors (96 vs 51%; P=0.001, log-rank test; Figure 3a). The same significant differences in the overall survival rate between the two groups of patients were also observed in combined stages I and II (P=0.020, Figure 3b) and stages III cases (P=0.01, Figure 3c). The 3-year survival rate of HLA-G negative and positive cases in stages II and I were 100 and 61%, respectively. In stage III, the survival rate was 82 and 42%, respectively. Stage IV was not considered because of the limited number of cases (n=4).
To compare with other clinicopathological factors, the effect of age, gender, depth of invasion, histological grade, nodal status and stages of the disease on the patients’ survival were also analyzed. As shown in Table 4, only the factors of nodal status and disease stages had a significant effect on the overall survival rate.
Univariate survival analysis was performed to investigate possible prognostic impact of HLA-G in colorectal cancer. As evident in Table 5, the expression of HLA-G correlated with a worsening of the survival probability, which was statistically significant. This was also confirmed by a multivariate survival analysis including age, gender, depth of invasion, histological grade, nodal status, stages of the disease and HLA-G status. All of these analyses suggest that HLA-G expression in tumor cells is an independent prognostic factor for colorectal cancer patients (relative risk=3.14; 95% confidence interval: 1.34–8.10, P=0.021).
Discussion
In this study, we have demonstrated that HLA-G protein was expressed in 65% of colorectal cancer specimens (Table 1). A number of other studies on epithelial tumors including colorectal cancer have been previously reported in which HLA-G protein expression determined by immunohistochemistry was seen in 54% of colorectal cancers,33 50–61% of ovarian cancers,9, 15 25% of invasive ductal breast cancers,9 61% of renal cell cancers,10 55% of endometrial adenocarcinoma16 and 52–79% in various gastrointestinal cancers.17 The different results obtained with the various types of malignancies may reflect the differences in the biology of the various types of tumors analyzed in the patient population, and/or it may reflect the sensitivity of the methods used to detect the HLA-G antigens in the malignant lesions. Furthermore, the regulation of HLA-G gene expression may differ among different tumor types, and it may be influenced by the tumor microenvironment, as well as by the pathogenesis underlying the malignant transformation of the cells.
Our results also revealed that epithelial cells of the normal colon–rectum or colorectal benign adenoma lacked any positive expression of the HLA-G antigen. The results are consistent with most other reported data34 and strongly support the notion that HLA-G expression is a highly specific marker for malignant transformation.9, 15, 16, 28
It has been hypothesized that one of the mechanisms for the aberrant re-expression of HLA-G in malignant lesions was due to the altered genomic controls during the process of malignant transformation.16 Thus, it can be further hypothesized that more aggressive cancers that have less organized genetic control may have more frequent HLA-G expression. Our study revealed that there was a statistically significant association between HLA-G expression and the various stages of colorectal cancer, where HLA-G positive staining was seen in 43% of stage I patients and in 70–71% of patients with stages II to IV (Tables 1, 2 and 3). We also found that a statistically significant correlation existed between HLA-G protein and the clinicopathological factors of depth of invasion, histological grade, host immune response and nodal status. However, the correlation did not extend to factors such as age or gender (Table 1). Similarly, other reports including Urosevic et al found that HLA-G immunoreactivity correlated with high-grade histology in the cancer of the lung.12 In advanced-stage ovarian cancer, HLA-G expression in effusions and metastases was significantly correlated,15 and a significant correlation was apparent with increasing HLA-G protein staining and increasing stages of endometrial adenocarcinoma.16 All of these findings together with our own suggest that HLA-G status might yield unfavorable prognosis for some types of epithelial cancer.
Currently, the TNM system represents the main tool for identifying prognostic differences among patients with colorectal cancer.4 The reported survival rate at 5 years is 90.5% for stage I patients, 67.9% for stages II and III and 9.4% for stage IV patients.35 In our prospective 3-year follow-up study, the overall survival rate was 88% for stage I patients, 87% for stage II and 57% for stage III patients (Table 4). However, we showed that there was a significant correlation between the overall survival rate and the HLA-G status regardless of the disease stages. The univariate and multivariate analyses performed also suggested that HLA-G status, along with the tumor stages and nodal status were strong predictors for the final clinical outcome (Table 5). All of our results lend credence to the idea that HLA-G status plays an important role in the clinical outcome of colorectal cancer. However, to fully substantiate the concept, further investigations with longer follow-up terms after the initial assay test are needed.
In colorectal cancer, the presence of many tumor associated antigens and their relationship with clinicopathological parameters have been described.36, 37 After surgery, adjuvant chemotherapy was indicated in patients with node metastasis. Although up to 40% of the patients with negative nodal status will eventually develop a recurrent disease during their lifetime, the role of adjuvant chemotherapy in this setting is still unclear.38 Identifying those patients with high-risk colorectal cancer would be of great benefit for improving the treatment strategies in the node-negative diseases.
Considering the immunomodulatory function as assigned to HLA-G molecules, its expression in malignant cells may represent one of various mechanisms used by the tumor cells to escape immune surveillance.34, 39 In this study, the finding of a significant reverse correlation that exists between HLA-G expression and host immune response provides further evidence to support the role that HLA-G plays in tumor escape mechanisms. As a result, this might grant them survival advantages over HLA-G negative tumors, which ultimately lead to unfavorable clinical outcomes.34 Besides altered genomic controls, the upregulation of HLA-G expression has also been proposed to be affected by tumor environmental factors, such as cytokines, stress and by agents used in chemotherapy, such as demethylating molecules.34 It is known that interferon-γ upregulates the expression of HLA-G in ovarian cancer and that this is associated with the resistance to lysis by peptide and allospecific CD8+ T cells.40 Therefore, the detection of HLA-G expression might be beneficial in planning adjuvant therapy, especially when an immunotherapy has already been planned. Furthermore, HLA-G in tumor cells has been seen to be significantly lower in effusions obtained during or following chemotherapy.15 This discovery might suggest that HLA-G status could be a valuable marker for monitoring chemotherapy as well.
In conclusion, we have demonstrated that HLA-G is expressed in majority of colorectal carcinomas. We have also established that HLA-G expression, as measured by immunohistochemistry in the primary tumor, has a strong and independent prognostic value in human colorectal cancer and therefore, making HLA-G protein staining a useful prognostic marker. Further investigations with a longer-term follow-up period will provide better insights into the role that HLA-G expression plays in tumor escape mechanisms.
References
Richi P, Zarrilli R, di Palma A, et al. Nonsteroidal anti-inflammatory drugs in colorectal cancer: from prevention to therapy. Br J Cancer 2003;88:803–807.
Ji BT, Devesa SS, Chow WH, et al. Colorectal cancer incidence trends by subsite in urban Shanghai, 1972–1994. Cancer Epidemiol Biomarkers Prev 1998;7:661–666.
You WC, Jin F, Devesa S, et al. Rapid increase in colorectal cancer rates in urban Shanghai, 1972–1997, in relation to dietary changes. J Cancer Epidemiol Prev 2002;7:143–146.
Chamberlain NL, Ward RL, Hawkins NJ . Clinicopathological significance of CERB-B2 expression in colorectal carcinoma. Oncol Rep 1999;6:527–531.
Kovats S, Main EK, Librach C, et al. A class I antigen, HLA-G, expressed in human trophoblasts. Science 1990;248:220–223.
Blaschitz A, Lenfant F, Mallet V, et al. Endothelial cells in chorionic fetal vessels of first trimester placenta express HLA-G. Eur J Immunol 1997;27:3380–3388.
Yang Y, Chu W, Geraghty DE, et al. Expression of HLA-G in human mononuclear phagocytes and selective induction by IFN-gamma. J Immunol 1996;156:4224–4231.
Crisa L, McMaster MT, Ishii JK, et al. Identification of a thymic epithelial cell subset sharing expression of the class Ib HLA-G molecule with fetal trophoblasts. J Exp Med 1997;186:289–298.
Singer G, Kurman RJ, McMaster MT, et al. HLA-G immunoreactivity is specific for intermediate trophoblast in gestational trophoblastic disease and can serve as a useful marker in differential diagnosis. Am J Surg Pathol 2002;26:914–920.
Ibrahim EC, Aractingi S, Allory Y, et al. Analysis of HLA antigen expression in benign and malignant melanocytic lesions reveals that upregulation of HLA-G expression correlates with malignant transformation, high inflammatory infiltration and HLA-A1 genotype. Int J Cancer 2004;108:243–250.
Bukur J, Rebmann V, Grosse-Wilde H, et al. Functional role of human leukocyte antigen-G up-regulation in renal cell carcinoma. Cancer Res 2003;63:4107–4111.
Urosevic M, Kurrer MO, Kamarashev J, et al. Human leukocyte antigen G up-regulation in lung cancer associates with high-grade, human histology, leukocyte antigen class I loss and interleukin-10 production. Am J Pathol 2001;159:817–824.
Lefebvre S, Antoinc M, Uzan S, et al. Specific activation of the non-classical class I histocompatibility HLA-G antigen and expression of the ILT2 inhibitory receptor in human breast cancer. J Pathol 2002;196:266–274.
Urosevic M, Willers J, Mueller B, et al. HLA-G protein up-regulation in primary cutaneous lymphomas is associated with interleukin-10 expression in large cell T-cell lymphomas and indolent B-cell lymphomas. Blood 2002;99:609–617.
Davidson B, Elstrand MB, McMaster MT, et al. HLA-G expression in effusions is a possible marker of tumor susceptibility to chemotherapy in ovarian carcinoma. Gynecol Oncol 2005;96:42–47.
Barrier BF, Kendall BS, Sharpe-Timms K, et al. Characterization of human leukocyte antigen-G (HLA-G) expression in endometrial adenocarcinoma. Gynecol Oncol 2006;103:25–30.
Hansel DE, Rahmanb A, Wilentz RE, et al. HLA-G upregulation in pre-malignant and malignant lesions of the gastrointestinal tract. Int J Gastrointest Cancer 2005;35:15–24.
Bainbridge DR, Ellis SA, Sargent IL . HLA-G suppresses proliferation of CD4 (+) T-lymphocytes. J Reprod Immunol 2000;48:17–26.
Lila N, Rouas-Freiss N, Dausset J, et al. Soluble HLA-G protein secreted by allo-specific CD4+ T cells suppresses the allo-proliferative response: a CD4+ T cell regulatory mechanism. Proc Natl Acad Sci USA 2001;98:12150–12155.
Wiendl H, Mitsdoerffer M, Hofmeister V, et al. A functional role of HLA-G expression in human gliomas: an alternative strategy of immune escape. J Immunol 2002;68:4772–4780.
Le Gal FA, Riteau B, Sedlik C, et al. HLA-G-mediated inhibition of antigen-specific cytotoxic T lymphocytes. Int Immunol 1999;11:1351–1356.
Riteau B, Menier C, Khalil-Daher I, et al. HLA-G1 co-expression boosts the HLA class I-mediated NK lysis inhibition. Int Immunol 2001;13:193–201.
LeMaoult J, Le Discorde M, Rouas-Freiss N, et al. Biology and functions of human leukocyte antigen-G in health and sickness. Tissue Antigens 2003;62:273–284.
Rebmann V, Regel J, Stolke D, et al. Secretion of sHLA-G molecules in malignancies. Semin Cancer Biol 2003;13:371–377.
Sebti Y, Le Friec G, Pangault C, et al. Soluble HLA-G molecules are increased in lymphoproliferative disorders. Hum Immunol 2003;64:1093–1101.
Nuckel H, Rebmann V, Durig J, et al. HLA-G expression is associated with an unfavorable outcome and immunodeficiency in chronic lymphocytic leukemia. Blood 2005;105:1694–1698.
Ugurel S, Rebmann V, Ferrone S, et al. Soluble human leukocyte antigen-G serum level is elevated in melanoma patients and is further increased by interferon-alpha immunotherapy. Cancer 2001;92:369–376.
Singer G, Rebmann V, Chen YC, et al. HLA-G is a potential tumor marker in malignant ascites. Clin Cancer Res 2003;9:4460–4464.
Herman P, Sobbing LH . UICC/TNM Classification of Malignant Tumors, 4th edn. Springer-Vela: New York, 1987, pp 47–49.
Harlow E, Lane D . Monoclonal antibodies. In: Harrow E, Lane D (eds). Antibodies: A Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory (NY), 1988, pp 139–243.
Kapasi K, Albert SE, Yie SM, et al. HLA-G has a concentration-dependent effect on the generation of an allo-CTL response. Immunology 2000;101:191–200.
Alexander J, Watanable T, Wu TT, et al. Histopathological identification of colon cancer with microsatellite instability. Am J Pathol 2001;158:527–535.
Fukushima Y, Oshika Y, Nakamura M, et al. Increased expression of human histocompatibility leukocyte antigen-G in colorectal cancer cells. Int J Mol Med 1998;2:349–351.
Rouas-Freiss N, Moreau P, Ferrone S, et al. HLA-G proteins in cancer: do they provide tumor cells with an escape mechanism? Cancer Res 2005;65:10139–10144.
Jemal A, Clegg LX, Ward E, et al. Annual report to the nation on the status of cancer, 1975–2001, with a special feature regarding survival. Cancer 2004;101:3–27.
Saucepan R, Funfair G, Secant A . Molecular prognostic factor in rectal cancer. Romanian J Gastroenterol 2004;3:223–231.
Graziano F, Cascinu S . Prognostic molecular markers for planning adjuvant chemotherapy trials in Duke's B colorectal cancer patients: how much evidence is enough? Ann Oncolo 2003;14:1026–1038.
Mcdonald JS . Adjuvant of therapy of colorectal cancer. CA Cancer J Clin 1999;49:202–219.
Algarra I, García-Lora A, Cabrera T, et al. The selection of tumor variants with altered expression of classical and nonclassical MHC class I molecules: implications for tumor immune escape. Cancer Immunol Immun 2004;53:904–910.
Malmberg KJ, Levitsky V, Norell H, et al. IFN-gamma protects short-term ovarian carcinoma cell lines from CTL lysis via a CD94/NKG2A-dependent mechanism. J Clin Invest 2002;110:1515–1523.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ye, Sr., Yang, H., Li, K. et al. Human leukocyte antigen G expression: as a significant prognostic indicator for patients with colorectal cancer. Mod Pathol 20, 375–383 (2007). https://doi.org/10.1038/modpathol.3800751
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800751
Keywords
This article is cited by
-
An exploration of immunohistochemistry-based prognostic markers in patients undergoing curative resections for colon cancer
BMC Cancer (2022)
-
HLA-G as a prognostic marker in stage II/III colorectal cancer: not quite there yet
Histochemistry and Cell Biology (2022)
-
HLA-G as a new tumor biomarker: detection of soluble isoforms of HLA-G in the serum and saliva of patients with colorectal cancer
Clinical and Translational Oncology (2020)
-
Serum and peritoneal fluid concentrations of soluble human leukocyte antigen, tumor necrosis factor alpha and interleukin 10 in patients with selected ovarian pathologies
Journal of Ovarian Research (2017)
-
Reduced expression of the murine HLA-G homolog Qa-2 is associated with malignancy, epithelial-mesenchymal transition and stemness in breast cancer cells
Scientific Reports (2017)