Abstract
Members of the Bcl-2 family of antiapoptotic proteins (Bcl-2, Bcl-XL and Mcl-1) are key regulators of apoptosis. The purpose of the present study was to examine and better define the role of Bcl-2, Bcl-XL and Mcl-1 in the progression of melanoma. Immunohistochemical staining for Bcl-2, Bcl-XL and Mcl-1 was performed on paraffin sections of 100 cases of benign nevi, primary melanoma and metastatic melanoma. Expression was correlated with histopathologic features, clinical progress and expression of transcription factors (AP-2, MITF and p-Stat3). Bcl-2 was expressed in 100% of benign nevi and thin melanoma (≤1.0 mm) but was less in thick melanoma (>1.0 mm) (88%), subcutaneous (62%) and lymph node metastases (35%). In contrast, Bcl-XL and Mcl-1 were expressed at lower levels in nevi and thin melanoma compared to Bcl-2 but their expression was much higher in thick melanoma and in subcutaneous and lymph node metastases (P<0.0001). Bcl-2 expression was negatively associated with tumor thickness (P<0.05) but Bcl-XL expression increased with increasing tumor thickness (P<0.05) and dermal tumor mitotic rate (P<0.05). Similarly Mcl-1 expression increased with increasing tumor thickness (P<0.09) and dermal tumor mitotic rate (P<0.17). Bcl-2 expression was positively correlated with expression of the transcription factors microphthalmia transcription factor (MITF) and nuclear AP-2 whereas Bcl-XL (and Mcl-1) expression were positively correlated with p-Stat3. This study is the first to show a clear dissociation between changes in Bcl-2 expression (downregulation) and Bcl-XL, Mcl-1 expression (upregulation) during progression of melanoma. The results were also consistent with a role for AP-2 and MITF in regulation of Bcl-2 and pStat3 in regulation of Bcl-XL. These findings have important implications for the development of treatments targeting antiapoptotic proteins in patients with melanoma.
Similar content being viewed by others
Main
It is now widely believed that the development of resistance to apoptosis is a crucial step in tumorigenesis and chemotherapeutic drugs resistance. Members of the Bcl-2 (B-cell lymphoma 2) family of proteins are key regulators of apoptosis.1 They include Bcl homology domain 3 (BH3) only sensor proteins (such as Bid, Bim, Bad, Noxa and PUMA) and antiapoptotic prosurvival proteins such as Bcl-2, Bcl-2-like 1 (Bcl-XL) and Myeloid leukemia-1 (Mcl-1) proteins. Bcl-2 associated X protein (Bax) and Bak are additional proapoptotic proteins that appear to be essential for induction of apoptosis.2, 3 The precise interactions between the Bcl-2 proteins leading to apoptosis remain uncertain but two BH3 proteins, Bid and Bim, appear to directly activate Bax and Bak and bind to mitochondria.4 The antiapoptotic proteins are believed to bind the proapoptotic BH3 proteins and so prevent their interaction with Bax and Bak and subsequent changes in mitochondria. Recent studies have also suggested that there may be some selectivity of the proapoptotic BH3 only proteins for particular antiapoptotic proteins for example Noxa appears to bind to Mcl-1 and Bcl-XL but not Bcl-2.5, 6
Bcl-2 was originally identified as a proto-oncogene in follicular B-cell lymphoma. In the lymphoma cells the Bcl-2 gene was found to be activated via a translocation between chromosome 18 and chromosome 14, where the gene comes controlled by the immunoglobulin heavy chain promoter.7 Mcl-1 was first reported in differentiating myeloid cells.8 As then Mcl-1 has been shown to be expressed in multiple cell lineages, and high levels of Mcl-1 were detected in epithelia such as in prostate, breast, colon and lung.9 Bcl-XL is the protein product of the Bcl-X gene. The protein product of the longer Bcl-X mRNA (Bcl-XL) becomes as a repressor of apoptosis,10, 11 whereas the shorter form (Bcl-XS) can enhance apoptosis. Bcl-XL is widespread in many solid tumors and is associated with enhanced cell survival and drug resistance.12, 13, 14, 15, 16, 17, 18
The role of these proteins in the progression of melanoma is uncertain.19, 20, 21, 22, 23 Bcl-2 expression in melanocytes has been reported to protect melanocyte stem cells from apoptosis, as shown by loss of pigmentation in Bcl-2 knockout mice and graying of hair in mice.24, 25 There are conflicting reports on the expression of the Bcl-2 family of proteins and other antiapoptotic prosurvival proteins in melanoma. Ramsay et al26 reported immunohistochemical detection of Bcl-2 in 100% of melanocytes and benign nevi, but found less expression in primary and metastatic melanoma. Similar results were reported by Saenz-Santamaria et al,27 Collins et al28 and Cerroni et al.29 The role of the other antiapoptotic proteins, Bcl-XL and Mcl-1 in melanoma is largely unknown. In 1998, Tang et al20 reported that Bcl-2 was downregulated in melanoma but Bcl-XL and Mcl-1 were upregulated. Leiter19 reported increased detection of Bcl-2 and Bcl-XL in advanced melanoma. Others have reported an association between Bcl-XL and resistance to chemotherapy, and have identified it as a target for antisense therapy.21, 22
Regulation of the antiapoptotic proteins by transcription factors is poorly understood but microphthalmia transcription factor (MITF), activating protein 2α (AP-2α) and phosphorylated signal transducer and activator of transcription 3 (pStat3) have been implicated in their control. In the present study, we sought to better characterize the association of the antiapoptotic proteins with progression of melanoma and to examine changes in transcription factors that may be involved in their regulation.
Materials and methods
Patients
The archival paraffin tissue blocks of 100 melanocytic tumors excised during the period from 2000 to 2003 were retrieved from the Department of Anatomical Pathology at the Royal Prince Alfred Hospital, Sydney, Australia. The study has ethics approval from the Sydney South West Area Health Service NSW Health Ethics Review Committee of Royal Prince Alfred Hospital (Protocol No X04-0061). There were (1) 10 compound nevi, (2) 10 dysplastic nevi, (3) 42 primary cutaneous melanoma including 18 thin primary cutaneous melanoma (≤1.0 mm in thickness), 24 thick primary cutaneous melanoma (>1.0 mm in thickness), (4) 21 subcutaneous (SC) melanoma metastases including one metastasis in skeletal muscle and (5) 17 lymph node (LN) metastases. The 42 patients with primary cutaneous melanoma were treated at the Sydney Melanoma Unit from December 2000 to March 2002. Hematoxylin and eosin (H&E) stained sections of all 100 cases were reviewed. Primary melanoma was classified into thin melanoma (≤1.0 mm) and thick melanoma (>1.0 mm) according their Breslow thickness. There were 18 nodular melanoma (NM), 14 superficial spreading melanoma (SSM), four desmoplastic melanoma, three in situ melanoma, two acral lentiginous melanoma and one lentigo maligna melanoma (melanoma arising in a Hutchinson's melanotic freckle). All patients with primary melanoma had regular followed-up at the Sydney Melanoma Unit (mean 43.5 months, range 21–50 months).
Immunohistochemistry
Five micrometer-thick sections were cut from the formalin-fixed, paraffin-embedded block of each case. Sections were deparaffinized in xylene and rehydrated through graded decreasing concentrations of alcohol. Antigen retrieval was carried out as reported previously30 in EDTA buffer pH 8.6 by heating in a microwave oven for 5 min and repeating this three times or in EDTA buffer pH 9.0 by heating in pressure-cooker for 4 min at 125°C (0.01 mol/l of citrate buffer pH 6.0 microwave antigen retrieval was used in place of the EDTA buffer in studies on Mcl-1). Anti-human Bcl-2 mouse antibody (monoclonal IgG, DAKO, Carpinteria, CA, USA, Cat M0887, clone 124) was added at a dilution of 1:50 in Tris buffer for 1 h. Rabbit anti-mouse immunoblubin (DAKO, Carpinteria, CA, USA) was used to detect the primary antibodies and was visualized using ‘ENVISION’-PLUS-HRP (Rabbit, DAKO, Carpinteria, CA, USA, Cat K4003) and DAB kit (DAKO, Carpinteria, CA, USA). Mouse anti-human Bcl-XL (H-5) antibody (monoclonal IgG1, Santa Cruz, San Diego, CA, USA, Cat SC-8392) was added at 1:400 dilution in Tris buffer and rabbit anti-human Mcl-1 antibody (polyclonal IgG, DAKO, Carpinteria, CA, USA, Lot 057) at a dilution of 1:30 in Tris buffer was incubated on the sections at room temperature for 1 h. Mouse anti-human MITF antibody (monoclonal IgG, DAKO, Carpinteria, CA, USA, Cat M3621, clone D5) at a dilution of 1:200 in Tris buffer, mouse anti-human pStat3 (B-7) antibody (monoclonal IgG2b, Santa Cruz, San Diego, CA, USA, Cat SC-8059) at dilution of 1:50 in Tris buffer and rabbit anti-human AP-2α (C-18) (polyclonal IgG, Santa Cruz, San Diego, CA, USA, Cat SC-184) at 1:300 dilution in Tris buffer were incubated on the sections at room temperature for 1 h. The Vectastain ABC kit (Vector Laboratories, Burlingame, CA, USA) was used to bind the antibodies according to the manufacturer's instructions and the binding sites were visualized using the DAB kit (DAKO, Carpinteria, CA, USA, Cat K3466). The sections were counterstained with Harris hematoxylin. Negative controls were performed by omission of the primary antibody and use of normal mouse IgG1 (DAKO, Carpinteria, CA, USA) in each experiment. Lymph node, normal breast tissue, prostate carcinoma and benign nevi were used as positive controls as appropriate.31, 32
The slides were examined by two investigators. The percentage of positive cells was estimated from 0 to 100%. Intensity of staining (intensity score) was judged on an arbitrary scale of 0 to 4+: no staining (0), weakly positive staining (1+), moderately positive staining (2+), strongly positive staining (3+) and very strongly positive staining (4+). An immunoreactive score (IRS) was derived by multiplying the percentage of positive cells with staining intensity divided by 10. The distribution pattern of staining was classified into focal or diffuse types. Localization of staining (nuclear, membranous or cytoplasmic) was also recorded.
Statistical Analysis
Statistical analysis was carried out using Microsoft Excel 2000 software and ‘JMP Statistics Made Visual™’ software (SAS Institute Inc., Trunbull, CT, USA). The one-way ANOVA two-tailed t-test was used to assess differences in expression of Bcl-2, Bcl-XL and Mcl-1 between different groups of melanocytic tumors. Multiple comparisons for all pairs were performed using the Tukey–Kramer HSD method and Student's t-test was also used for all pairs. The comparisons of the Bcl-2, Bcl-XL and Mcl-1 expression in histopathologic subtypes of primary melanoma excluding acral lentiginous melanoma and the lentigo maligna melanoma were performed using the one-way ANOVA two-tailed t-test and the Tukey-Kramer HSD method. The correlations between Bcl-2, Bcl-XL and Mcl-1 expression in primary melanoma and Breslow thickness and dermal mitotic rate were assessed using regression analysis. Correlations between Bcl-2, Bcl-XL, Mcl-1 expression and MITF, AP-2α, pStat3 expression in melanocytic lesions were also examined by regression analysis.
Disease free survival (DFS) and overall survival (OS) were calculated using Kaplan–Meier univariate estimates. Differences in DFS and OS between patients with melanoma according to tumor thickness (≤1 mm thickness and >1 mm thickness) and Bcl-2, Bcl-XL and Mcl-1 expression (above and below the median percentage value or above and below the median IRS score) were compared using log-rank and χ2 statistical methods, respectively. The univariate analysis was followed by multivariate analysis according to the Cox Proportional Hazards Model using SPSS software. A P<0.05 was considered statistically significant.
Results
Melanocytic Lesions Studied
The melanocytic lesions studied and the corresponding patient demographics are summarized in Table 1. The age of the patients ranged from 16 to 93 years with a median of 66 years. Thirty-eight patients were female patients and 62 were male patients. The age of patients with primary melanoma ranged from 23 to 84 years with a median age of 69 years. Fourteen of the latter group of patients were female patients (33%) and 28 were male patients (67%). The Breslow thickness of the primary melanoma ranged from 0.1 to 16 mm. Eighteen were ≤1 mm in thickness and 24 were >1 mm. The majority of the melanoma was of the nodular (18) or the superficial spreading (14) histological subtypes. (Owing to depletion of the samples of thin melanoma, one case was excluded in studies on Bcl-2 and nine cases in studies on the transcription factors.)
Bcl-2 Expression
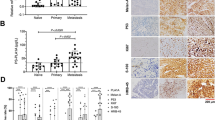
As shown in Table 2, Bcl-2 was extensively expressed in all compound and dysplastic nevi and in thin (≤1.0 mm) melanoma. Expression was, however, less in thick melanoma (>1.0 mm) (88%), subcutaneous metastases (62%) and lymph node metastases (35%). Bcl-2 expression was located in the cytoplasm of the cells. It was also identified in melanocytes and lymphocytes. (Expression in the latter was used as a positive internal control.) Intensity of staining varied from negative to 4+. It was strongest in compound nevi, dysplastic nevi and primary melanoma ≤1 mm in thickness. Expression was much weaker in thick primary melanoma (>1 mm) and metastases (Figure 1Aa–Ac). The distribution of staining was diffuse in benign nevi and primary melanoma ≤1 mm in thickness and diffuse or focal in melanoma >1 mm in thickness and metastases. The differences shown in Figure 2a were significant using the one-way ANOVA two-tailed t-test (P<0.0001).
Bcl-2 family proteins and transcription factors expression in different types of melanocytic tumors. (A) Bcl-2: (a) Compound nevus (× 400); positive cells: 95%; IRS=19. (b) Melanoma >1.0 mm thick (× 400); positive cells: 95%; IRS=9.5. (c) Subcutaneous metastasis negative; (× 400). (B) Bcl-XL: (a) Compound nevus negative; (× 400). (b) Melanoma >1.0 mm thick (× 400); positive cells: 98%; IRS=29.4. (c) Subcutaneous metastasis (× 400); positive cells: 85%; IRS=12.75. (C) Mcl-1: (a) Compound nevus (× 400); positive cells: 80%; IRS=4. (b) Melanoma >1.0 mm thick (× 400); positive cells: 95%; IRS=28.5. (c) Subcutaneous metastasis (× 400); positive cells: 95%; IRS=28.5. (D) pStat3: (a) Compound nevus nuclear negative; (× 400). (b) Melanoma >1.0 mm thick (× 400); positive cells: 20%; IRS=4. (c) Subcutaneous metastasis (× 400); positive cells: 50%; IRS=15. (E) AP-2α: (a) Compound nevus (× 400); Nuclear positive cells: 100%; IRS=40. Melanoma >1.0 mm thick (× 400); Nuclear positive cells: (b) 30%; IRS=7.5. (c) Subcutaneous metastasis nuclear negative; (× 400). (F) MITF: (a) Compound nevus (× 400); Nuclear positive cells: 100%; IRS=40. (b) Melanoma >1.0 mm thick (× 400); Nuclear positive cells: 100%; IRS=40. (c) Subcutaneous metastasis nuclear negative; (× 400).
(a) Mean immunoreactive score of positive cells for Bcl-2, Bcl-XL and Mcl-1 in different types of melanocytic lesions. N=nevus; M=melanoma; Met=metastatic; LN=lymph node; n=number; thick and thin=Breslow thickness >1.0 mm and ≤1.0 mm; IRS=immunoreactive score; Y bar indicates one s.e. Statistical differences by Student's t-test and Tukey Kramer tests were as follows: Bcl-2: *CN vs SC met P<0.05 (Student's t-test only), vs LN met P<0.0001; **DN vs SC met P<0.005, vs LN met P<0.0001; ***Thin M vs CN P=0.005 (Student's t-test only), vs thick M P<0.001, vs SC met and LN met P<0.0001. Bcl-XL: *Thick M vs thin M P<0.005, vs DN P=0.0005, vs CN P<0.0001; **SC met vs thick M P<0.05, vs thin M, DN and CN P<0.0001; ***LN met vs thick M P<0.01, vs thin M, DN and CN P<0.0001. Mcl-1: *Thick M vs thin M and DN P<0.05, vs CN P<0.005 (Student's t-test only); **SC met vs thick M P<0.05 (Student's t-test only), vs thin M P<0.0005, vs DN and CN P<0.0001; ***LN met vs thick M P<0.05 (Student's t-test only), vs thin M P<0.0005, vs DN and CN P<0.0001. (b) Mean immunoreactive score of positive cells for Bcl-2, Bcl-XL and Mcl-1 in different histopathologic types of primary melanoma. n=number; IRS=immunoreactive score; Bcl-2: *Desmoplastic melanoma vs melanoma in situ P=0.005, vs superficial spreading P<0.01 (Student's t-test and Tukey Kramer); Bcl-XL: **Nodular melanoma vs superficial spreading P=0.003; vs melanoma in situ P=0.015 (Student's t-test and Tukey Kramer).
Bcl-XL and Mcl-1 Expression
In contrast to Bcl-2 expression, Bcl-XL and Mcl-1 expression was less in benign pigmented lesions and thin melanoma compared to that in thick melanoma (>1.0 mm) and metastases (Table 2, Figure 1Ba–Bc, Ca–Cc). Bcl-XL and Mcl-1 were expressed in the cytoplasm of the cells. The distribution of staining was diffuse or focal. Bcl-XL was not detectable in adjacent normal melanocytes but was positive in plasma cells. Staining of normal breast tissue was used as a positive control where it was localized to ductal epithelium. Mcl-1 was detected in lymphocytes. The intensity of staining was less in compound nevi, dysplastic nevi and melanoma ≤1 mm in thickness than it was in melanoma >1 mm in thickness and metastases. Its distribution was predominantly diffuse. The differences shown in Figure 2a were significant using the one-way ANOVA two-tailed t-test (P<0.0001).
Bcl-2, Bcl-XL and Mcl-1 expression in relation to histopathologic subtypes of melanoma is shown in Figure 2b. There was a higher mean immunoreactive score of Bcl-2 positive cells in superficial spreading melanoma (IRS=23.9) compared to that seen in nodular melanoma (IRS=15.4) (Student's t-test P=0.02) and desmoplastic melanoma (IRS=4.6) (Student's t-test and Tukey–Kramer P=0.001). In contrast, mean immunoreactive score of Bcl-XL positive cells in superficial spreading melanoma was lower (IRS=6.7) than that seen in nodular melanoma (IRS=16.2) (Student's t-test and Tukey–Kramer P=0.003). Similar trends were seen in expression of Mcl-1 and histopathologic subtypes (one way ANOVA P=0.24).
Expression of Transcription Factors
As shown in Table 2 and Figure 1 Da-Dc, pStat3 was not detected in benign nevi and melanoma ≤1 mm in thickness and was variably found in thick melanoma (>1.0 mm) and in metastases. In contrast, AP-2α was strongly expressed in the nuclei of benign nevi and thin melanoma. Expression was much less in melanoma >1.0 mm thick, subcutaneous and lymph node metastases. AP-2α was detected in the cytoplasm of all melanoma but only infrequently in benign pigmented lesions (Figure 1Ea–Ec). MITF was strongly expressed in the nuclei of benign nevi and thin melanoma but was less in melanoma >1.0 mm in thickness, subcutaneous and lymph node metastases (Figure 1Fa–Fc).
Relation of Bcl-2, Bcl-XL and Mcl-1 Expression to Known Prognostic Features in Primary Melanoma
As shown in Figure 3a and b, Bcl-2 expression was inversely associated with tumor thickness (P=0.0013) and dermal tumor mitotic rate (P=0.33). In contrast, Bcl-XL expression was increased with increased tumor thickness (P=0.016) and with increased dermal tumor mitotic rate (P=0.0024). Expression of Mcl-1 trended to increasing tumor thickness (P=0.0997) and with dermal tumor mitotic rate (P=0.17).
(a) Immunoreactive score of Bcl-2, Bcl-XL and Mcl-1 positive tumor cells in primary melanoma in relation to tumor thickness. Bcl-2 expression negatively associated with increased tumor thickness (R2=0.2355, P=0.0013) but Bcl-XL expression was positively associated with increased tumor thickness (R2=0.1402, P=0.0159) and Mcl-1 was an increased trend with increased tumor thickness (R2=0.068, P=0.0997). (b) Immunoreactive score of Bcl-2, Bcl-XL and Mcl-1 positive tumor cells in primary melanoma in relation to dermal tumor mitotic rate. Bcl-2 expression has an inverse trend associated with dermal tumor mitoses but Bcl-XL was positively associated with mitoses and Mcl-1 has a trend increasing with increased dermal mitoses.
Relation of Bcl-2, Bcl-XL and Mcl-1 Expression to the Expression of the Transcription Factors
The graphs in Figure 4a–c showed that Bcl-2 expression was positively associated with AP-2α (P<0.0001) and MITF nuclear expression (P=0.003), but was negatively associated with pStat3 expression. In contrast, Bcl-XL expression was negatively associated with AP-2α nuclear expression (P<0.0001) and positively associated with pStat3 expression (P=0.015). Mcl-1 expression was also negatively associated with AP-2α nuclear expression (P<0.0001) and was weakly associated with pStat3 expression (P=0.37).
DFS and OS in Relation to Bcl-2, Bcl-XL and Mcl-1 Expression
During follow-up of the patients with primary cutaneous melanoma (median follow up 43.5 months), 15 patients (36%) developed recurrent melanoma. Among those, five patients (11.9%) had locoregional recurrences, two patients (4.8%) had distant metastases and eight patients (19.0%) had both. Nine patients (21.4%) died with melanoma. No patients with melanoma ≤1 mm thickness had a recurrence or died. When patients were grouped above and below the median values for the percentage of melanoma cells positive or IRS for Bcl-2, Bcl-XL, Mcl-1 there was a significant relationship to DFS but not to OS. DFS was higher in the patients with 100% Bcl-2 expression (n=21) or IRS ≥20 (n=21) compared to patients with Bcl-2 expression <100% or IRS<20 (n=19) (χ2=5.75, P=0.017) (Figure 5a). In contrast, DFS was less in the patients with Bcl-XL above the median (70%) (n=19) compared to those below the median (n=21) (χ2=5.61, P=0.018) or with IRS scores above the median (≥10) (n=20) compared with patients with IRS below the median (<10) (n=20) (χ2=5.42, P=0.0198) (Figure 5b). Similarly, DFS was less in the patients with %Mcl-1 above median (60%) (n=21) compared with that of patients with Mcl-1 below 60% (n=19) (χ2=6.67, P=0.01) or with IRS above median (≥7) (n=20) compared to that in patients having IRS less than median (<7) (n=20) (χ2=7.98, P=0.0047) (Figure 5c). When analyzed in the Cox Proportional Hazards Model with age, sex, Breslow thickness, Clark level, ulceration, mitotic rate, histopathologic subtypes, predominant cell type, vascular invasion and neural invasion, it was found that expression of the antiapoptotic proteins were not independent variables but were dependent largely on tumor thickness.
Kaplan–Meier estimates of DFS after excision of primary cutaneous melanoma. (a) DFS of patients with Bcl-2 positive cells IRS ≥20 vs patients with Bcl-2 positive cells IRS <20. (b) DFS of patients with Bcl-XL positive cells IRS ≥10 vs patients with Bcl-XL positive cells IRS <10. (c) DFS of patients with Mcl-1 positive cells IRS <7 vs patients with Mcl-1 positive cells IRS ≥7.
Discussion
The results of this study are consistent in part with several immunohistochemic and Western blot studies carried out by others26, 27, 29, 33 over the past decade and are contrary to the concept that Bcl-2 expression is associated with progression of melanoma. Instead, our results show that Bcl-2 expression was associated with a good prognosis, as reported by Divito et al34 in studies on tissue microarrays. The importance of clarifying Bcl-2 expression in melanomas has been heightened by recent clinical studies that have attempted to reduce Bcl-2 levels by antisense approaches based on the (false) assumption that Bcl-2 levels are uniformly high in melanoma.35 Our studies show that the majority of metastases in lymph nodes are completely negative for Bcl-2 expression and even when present it is at low levels. Similarly, expression in subcutaneous metastases is low, with most cells being negative for Bcl-2. The validity of these results is borne out by staining of lymphocytes for Bcl-2 in the sections.
Reduction in Bcl-2 expression was evident in thick melanoma and in melanoma with high dermal mitotic rates, suggesting that it was related to events associated with disease progression. In view of this, we investigated the expression of certain transcription factors. One of these is the microphthalmia associated transcription factor (MITF) that is believed to be responsible for differentiation and survival of melanocytes36 and a key factor in regulation of Bcl-2. MITF is regulated through C-kit and C-kit is downregulated in melanoma cells.37 This may therefore play some role in the decreased levels of Bcl-2 via decreased activation of MITF. Regression analysis of the present data showed a correlation between low levels of MITF and Bcl-2 in melanoma.
Another transcription factor regulating Bcl-2 (and C-kit) is AP-2.38 This was previously shown to be lost in progression of melanoma and loss of AP-2 was associated with short OS and DFS.39 Reports by others have suggested that translocation of AP-2 from the cytoplasm to the nucleus is disrupted during melanoma progression and is a crucial event in the development of melanoma.40 AP-2 may act to regulate not only Bcl-2 expression but also several other genes whose products are involved in tumor growth and metastasis of melanoma. AP-2 proteins were reported to bind with p53 to p53 target genes such as p21 and so act as a tumor suppressor. It is not clear why AP-2 nuclear expression is lost in melanoma but AP-2 appears essential for development of neural crest lineages.38 In the present study, there was a strong correlation between low levels of AP-2 and Bcl-2 consistent with a direct relation between AP-2 and Bcl-2.
In contrast to Bcl-2, Mcl-1 and Bcl-XL expressions are consistent with a role in the progression of melanoma. Mcl-1 expression has received relatively little attention in melanoma but was reported to be overexpressed in a number of other cancers.41, 42, 43, 44 Moreover, antisense knockdown of Mcl-1 sensitized melanoma xenografts to chemotherapy with dacarbazine.45 The regulation of Mcl-1 protein expression in melanoma is poorly understood. Betulinic acid has been shown to upregulate Mcl-1 in melanoma cells by activation of Akt and MAP kinase pathways.46 Activation of Stat3 by Src kinases in melanoma cells resulted in upregulation of both Mcl-1 and Bcl-XL47 and Stat3 was regarded as a critical transcriptional activator of Mcl-1, Bcl-XL and survivin.48 The present results are consistent with a role for activated Stat3 in upregulation of Bcl-XL and Mcl-1 although correlation with the latter was not statistically significant. Mcl-1 levels in melanoma were downregulated by the multi-kinase inhibitor BAY 43-9006 (Sorafenib) but this was apparently due to increased proteasome degradation49 perhaps by the specific Mcl-1 ubiquitin ligase E3 (Mule).50
Bcl-XL expression suggested that this protein was regulated differently to Bcl-2. The Bcl-X gene contains several tissue-specific transcription start sites and multiple consensus binding sites for both ubiquitous and tissue-specific transcription factors51 and its regulation is therefore likely to be complex. It was shown to be a downstream target of Stat3 and Stat5.48 A positive correlation was shown in the present study between pStat3 and Bcl-XL expression but it is highly likely that other transcription factors such as NF-κB are involved in regulation of Bcl-XL.52 AP-2 expression was negatively associated with Bcl-XL and Mcl-1 expression but further studies are needed to confirm whether the associations shown are causally related.
In summary, this study has shown a dissociation between Bcl-2 compared to Bcl-XL and Mcl-1 expression during progression of melanoma. These changes were significantly associated in univariate analysis with decreased DFS but not in multivariate survival analysis. Changes in Bcl-2 were strongly associated with changes in the transcription factors AP-2α and MITF, suggesting that changes in these factors may be responsible for changes in Bcl-2. Similarly, increases in pStat3 were associated with increases in Bcl-XL and (to a lesser extent) Mcl-1 expression, consistent with a role for pStat3 in upregulation of these proteins. These results have importance in pointing to treatment targets in patients with metastatic melanoma.
References
Cory S, Adams JM . The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer 2002;2:647–656.
Cheng EH, Wei MC, Weiler S, et al. BCL-2, BCL-X(L) sequester BH3 domain-only molecules preventing BAX- and BAK-mediated mitochondrial apoptosis. Mol Cell 2001;8:705–711.
Wei MC, Zong WX, Cheng EH, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 2001;292:727–730.
Letai A, Bassik MC, Walensky LD, et al. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell 2002;2:183–192.
Willis SN, Chen L, Dewson G, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev 2005;19:1294–1305.
Chen L, Willis SN, Wei A, et al. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 2005;17:393–403.
Tsujimoto Y, Cossman J, Jaffe E, et al. Involvement of the bcl-2 gene in human follicular lymphoma. Science 1985;228:1440–1443.
Kozopas KM, Yang T, Buchan HL, et al. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci USA 1993;90:3516–3520.
Krajewski S, Bodrug S, Krajewska M, et al. Immunohistochemical analysis of Mcl-1 protein in human tissues. Differential regulation of Mcl-1 and Bcl-2 protein production suggests a unique role for Mcl-1 in control of programmed cell death in vivo. Am J Pathol 1995;146:1309–1319.
Boise LH, Gonzalez-Garcia M, Postema CE, et al. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 1993;74:597–608.
Nunez G, Merino R, Simonian PL, et al. Regulation of lymphoid apoptosis by Bcl-2 and Bcl-XL. Adv Exp Med Biol 1996;406:75–82.
Olopade OI, Adeyanju MO, Safa AR, et al. Overexpression of BCL-x protein in primary breast cancer is associated with high tumor grade and nodal metastases. Cancer J Sci Am 1997;3:230–237.
Simoes-Wust AP, Olie RA, Gautschi O, et al. Bcl-xl antisense treatment induces apoptosis in breast carcinoma cells. Int J Cancer 2000;87:582–590.
Liu JR, Fletcher B, Page C, et al. Bcl-xL is expressed in ovarian carcinoma and modulates chemotherapy-induced apoptosis. Gynecol Oncol 1998;70:398–403.
Lebedeva I, Rando R, Ojwang J, et al. Bcl-xL in prostate cancer cells: effects of overexpression and down-regulation on chemosensitivity. Cancer Res 2000;60:6052–6060.
Luo D, Cheng SC, Xie H, et al. Effects of Bcl-2 and Bcl-XL protein levels on chemoresistance of hepatoblastoma HepG2 cell line. Biochem Cell Biol 2000;78:119–126.
Leech SH, Olie RA, Gautschi O, et al. Induction of apoptosis in lung-cancer cells following bcl-xL anti-sense treatment. Int J Cancer 2000;86:570–576.
Dole MG, Jasty R, Cooper MJ, et al. Bcl-xL is expressed in neuroblastoma cells and modulates chemotherapy-induced apoptosis. Cancer Res 1995;55:2576–2582.
Leiter U, Schmid RM, Kaskel P, et al. Antiapoptotic bcl-2 and bcl-xL in advanced malignant melanoma. Arch Dermatol Res 2000;292:225–232.
Tang L, Tron VA, Reed JC, et al. Expression of apoptosis regulators in cutaneous malignant melanoma. Clin Cancer Res 1998;4:1865–1871.
Olie RA, Hafner C, Kuttel R, et al. Bcl-2 and bcl-xL antisense oligonucleotides induce apoptosis in melanoma cells of different clinical stages. J Invest Dermatol 2002;118:505–512.
Heere-Ress E, Thallinger C, Lucas T, et al. Bcl-X(L) is a chemoresistance factor in human melanoma cells that can be inhibited by antisense therapy. Int J Cancer 2002;99:29–34.
Li W, Sanki A, Karim R, et al. The role of cell cycle regulatory proteins in the pathogenesis and prognosis of melanoma. Pathology 2006;38:287–301.
Nishimura EK, Granter SR, Fisher DE . Mechanisms of hair graying: incomplete melanocyte stem cell maintenance in the niche. Science 2005;307:720–724.
Bouillet P, Cory S, Zhang LC, et al. Degenerative disorders caused by Bcl-2 deficiency prevented by loss of its BH3-only antagonist Bim. Dev Cell 2001;1:645–653.
Ramsay JA, From L, Kahn HJ . bcl-2 protein expression in melanocytic neoplasms of the skin. Mod Pathol 1995;8:150–154.
Saenz-Santamaria MC, Reed JA, McNutt NS, et al. Immunohistochemical expression of BCL-2 in melanomas and intradermal nevi. J Cutan Pathol 1994;21:393–397.
Collins KA, White WL . Intercellular adhesion molecule 1 (ICAM-1) and bcl-2 are differentially expressed in early evolving malignant melanoma. Am J Dermatopathol 1995;17:429–438.
Cerroni L, Soyer HP, Kerl H . bcl-2 protein expression in cutaneous malignant melanoma and benign melanocytic nevi. Am J Dermatopathol 1995;17:7–11.
Shi S-R, Gu J, Taylor CR (eds). Antigen Retrieval Techniques: Immunohistochemistry and Molecular Morphology. Eaton Publishing: Natrick, 2000, pp 311–320.
Krajewski S, Krajewska M, Shabaik A, et al. Immunohistochemical analysis of in vivo patterns of Bcl-X expression. Cancer Res 1994;54:5501–5507.
Horinaga M, Okita H, Nakashima J, et al. Clinical and pathologic significance of activation of signal transducer and activator of transcription 3 in prostate cancer. Urology 2005;66:671–675.
Tron VA, Krajewski S, Klein-Parker H, et al. Immunohistochemical analysis of Bcl-2 protein regulation in cutaneous melanoma. Am J Pathol 1995;146:643–650.
Divito KA, Berger AJ, Camp RL, et al. Automated quantitative analysis of tissue microarrays reveals an association between high Bcl-2 expression and improved outcome in melanoma. Cancer Res 2004;64:8773–8777.
Bedikian A, Millward M, Pehamberger H, et al, Group tOMS. Bcl-2 antisense (Oblimersen sodium) plus dacarbazine in patients with advanced melanoma. J Clin Oncol 2006;24:4738–4745.
McGill GG, Horstmann M, Widlund HR, et al. Bcl2 regulation by the melanocyte master regulator Mitf modulates lineage survival and melanoma cell viability. Cell 2002;109:707–718.
Janku F, Novotny J, Julis I, et al. KIT receptor is expressed in more than 50% of early-stage malignant melanoma: a retrospective study of 261 patients. Melanoma Res 2005;15:251–256.
Leslie MC, Bar-Eli M . Regulation of gene expression in melanoma: new approaches for treatment. J Cell Biochem 2005;94:25–38.
Karjalainen JM, Kellokoski JK, Eskelinen MJ, et al. Downregulation of transcription factor AP-2 predicts poor survival in stage I cutaneous malignant melanoma. J Clin Oncol 1998;16:3584–3591.
Berger AJ, Davis DW, Tellez C, et al. Automated quantitative analysis of activator protein-2alpha subcellular expression in melanoma tissue microarrays correlates with survival prediction. Cancer Res 2005;65:11185–11192.
Wuilleme-Toumi S, Robillard N, Gomez P, et al. Mcl-1 is overexpressed in multiple myeloma and associated with relapse and shorter survival. Leukemia 2005;19:1248–1252.
Song L, Coppola D, Livingston S, et al. Mcl-1 regulates survival and sensitivity to diverse apoptotic stimuli in human non-small cell lung cancer cells. Cancer Biol Ther 2005;4:267–276.
Sano M, Nakanishi Y, Yagasaki H, et al. Overexpression of anti-apoptotic Mcl-1 in testicular germ cell tumors. Histopathology 2005;46:532–539.
O'Driscoll L, Cronin D, Kennedy SM, et al. Expression and prognostic relevance of Mcl-1 in breast cancer. Anticancer Res 2004;24:473–482.
Thallinger C, Wolschek MF, Wacheck V, et al. Mcl-1 antisense therapy chemosensitizes human melanoma in a SCID mouse xenotransplantation model. J Invest Dermatol 2003;120:1081–1086.
Selzer E, Thallinger C, Hoeller C, et al. Betulinic acid-induced Mcl-1 expression in human melanoma--mode of action and functional significance. Mol Med 2002;8:877–884.
Niu G, Bowman T, Huang M, et al. Roles of activated Src and Stat3 signaling in melanoma tumor cell growth. Oncogene 2002;21:7001–7010.
Jing N, Tweardy DJ . Targeting Stat3 in cancer therapy. Anticancer Drugs 2005;16:601–607.
Yu C, Bruzek LM, Meng XW, et al. The role of Mcl-1 downregulation in the proapoptotic activity of the multikinase inhibitor BAY 43-9006. Oncogene 2005;24:6861–6869.
Zhong Q, Gao W, Du F, et al. Mule/ARF-BP1, a BH3-only E3 ubiquitin ligase, catalyzes the polyubiquitination of Mcl-1 and regulates apoptosis. Cell 2005;121:1085–1095.
Grillot DA, Gonzalez-Garcia M, Ekhterae D, et al. Genomic organization, promoter region analysis, and chromosome localization of the mouse bcl-x gene. J Immunol 1997;158:4750–4757.
Amiri KI, Richmond A . Role of nuclear factor-kappa B in melanoma. Cancer Metastasis Rev 2005;24:301–313.
Acknowledgements
We thank the staff of the Department of Anatomical Pathology, Royal Prince Alfred Hospital, especially Stewart Davies, Dianne Maguire and Trina Lum, for their assistance with immunohistochemistry. We also thank the staff of the Oncology and Immunology Unit of the Newcastle Mater Misericordiae Hospital for their assistance and helpful comments and Marjorie Colman from the Sydney Melanoma Unit Database for follow up information. This study was supported in part by Funding from Melanoma and Skin Cancer Research Institute (MASCRI) and the National Health and Medical Research Council of Australia.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of interest
The authors disclose that there is no financial or personal conflict of interests amongst themselves and with industry.
Rights and permissions
About this article
Cite this article
Zhuang, L., Lee, C., Scolyer, R. et al. Mcl-1, Bcl-XL and Stat3 expression are associated with progression of melanoma whereas Bcl-2, AP-2 and MITF levels decrease during progression of melanoma. Mod Pathol 20, 416–426 (2007). https://doi.org/10.1038/modpathol.3800750
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800750