Abstract
Anti-calretinin antibodies are useful to differentiate adenocarcinomas from malignant mesotheliomas of the lung. Therefore, calretinin expression is rarely reported for sarcomatoid mesotheliomas. Anti-podoplanin antibodies (eg D2-40) react with lymphatic endothelia, Kaposi's sarcoma, lymphangioma and mesotheliomas. For the interpretation of spindle cell lesions of the pleura, knowledge of calretinin and D2-40 expression frequencies in sarcomatoid mesothelioma is desirable. To systematically investigate the sensitivity of calretinin and D2-40 antibodies in epithelioid and sarcomatoid areas of malignant mesotheliomas, a tissue microarray with 341 malignant mesotheliomas, including 112 epithelioid, 46 sarcomatoid and 183 biphasic tumors was constructed. Epithelioid and sarcomatoid differentiated tumor areas were clearly separated within the tissue microarray. Expression of calretinin and D2-40 was separately studied in epithelioid and sarcomatoid areas by immunohistochemistry. Calretinin expression was found in 91% of epithelioid and 57% of sarcomatoid tumor areas. D2-40 immunostaining was present in 66% of the epithelioid and 30% of the sarcomatoid tumor areas. A combination of calretinin and D2-40 increased the sensitivity in epithelioid tumor areas to 0.96 and in sarcomatoid tumor areas to 0.66. These data indicate that a combination of calretinin and D2-40 will improve diagnostic accuracy for spindle cell lesions of the pleura, whereas almost all epithelioid mesotheliomas are identified by calretinin alone.
Similar content being viewed by others
Main
With its wide differential diagnosis and its therapeutical and prognostic implications, the accurate diagnosis of malignant mesothelioma is nowadays crucial. The most thoroughly investigated histologic mimic of malignant mesothelioma is adenocarcinoma of the lung. A variety of ancillary techniques including electron microscopy, histochemistry and immunohistochemistry assist in the differential diagnosis between malignant mesothelioma and adenocarcinoma. As surgical pathologists are confronted with an ever-decreasing size of biopsies, the necessity of a reliable marker is more and more apparent. Immunohistochemical panels are most promising and a wide range of sensitive and specific markers for lung adenocarcinoma have been described.1, 2, 3
Calretinin is a well-established immunohistochemical marker for malignant mesothelioma. It is a calcium-binding protein of the EF-hand family and has been shown to be of good discriminatory value in the distinction between epithelioid malignant mesothelioma and adenocarcinoma. Calretinin is expressed in both the cytoplasm and nucleus. According to the literature, its expression in malignant mesothelioma varies,3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 but most authors report frequent expression in epithelioid malignant mesothelioma, with only few studies reporting expression below 90%. Interestingly, calretinin expression has been described in a wide variety of cells, including steroid-producing cells of the testis and ovary, adipocytes, eccrine-glands, keratinizing thymic epithelial cells and numerous tumors, for example Merkel cell carcinoma, Leydig cell tumors of the testis, esthesioneuroblastoma, adenomas of the adrenal gland, small cell carcinoma of the lung and adenomatoid tumor.5, 21, 22
In addition to calretinin, D2-40 has been recently recommended as a new marker for malignant mesothelioma.23, 24 D2-40 is a novel monoclonal antibody that was reported to recognize a 40 kDa surface sialoglycoprotein expressed in germ cell neoplasia and fetal testicular gonocytes.25 A recent investigation has demonstrated that this mucin-type glycoprotein is podoplanin.26 Martín-Villar et al28 and Ma et al27 both described its human genome. D2-40 is selective for lymphatic endothelia and has been shown to react with Kaposi's sarcoma, lymphangioma and Dabska tumor.29, 30 Membranous staining of D2-40 has been described in 51 of 53 (96%)23 and 29 of 40 (72%) malignant mesotheliomas.24
No single antibody has demonstrated entire sensitivity or specificity for malignant mesothelioma,5, 22, 23, 31, 32, 33, 34 and an exact description of expression frequency in areas with sarcomatoid differentiation in sarcomatoid malignant mesothelioma or biphasic malignant mesothelioma has been largely neglected. The few publications that have evaluated calretinin expression in the three most frequent types of malignant mesothelioma separately present data that diverge from 18 to 100% positivity in sarcomatoid malignant mesothelioma, and 8 to 100% in biphasic malignant mesothelioma.5, 7, 15, 18, 19, 20, 33 The D2-40 staining frequency is also controversial in sarcomatoid and biphasic malignant mesothelioma. Whereas Chu et al23 reported staining in all types of malignant mesothelioma, Ordóñez24 did not detect any D2-40 staining in either sarcomatoid malignant mesothelioma or sarcomatoid areas of biphasic malignant mesothelioma.
With the availability of these novel mesothelioma markers, the differential diagnosis to adenocarcinoma has been facilitated in the last years. However, various spindle cell lesions of the pleura have broadened the differential diagnosis of sarcomatoid mesothelioma. These include especially synovial sarcomas of the pleura,35, 36, 37, 38, 39 but also solitary fibrous tumors, spindle cell carcinomas and high-grade sarcomas.
Consequently, we have constructed a tissue microarray with 341 malignant mesotheliomas and have clearly separated areas with epithelioid and sarcomatoid differentiation, in order to compare the value of D2-40 and calretinin immunostaining in distinct growth patterns of malignant mesothelioma. Tissue microarrays have proven highly representative in comparison with traditional evaluation through methods on large tissue samples.40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51
Materials and methods
Tumors
All malignant mesotheliomas, diagnosed between 1975 and 2004, were retrieved from the archives of the Zurich Pneumoconiosis Research Group, Switzerland (Director M Rueegger). The total of 341 cases comprised 112 epithelioid, 46 sarcomatoid and 183 biphasic types. All but three cases are pleural malignant mesotheliomas, one case had both pleural and peritoneal involvement and two were peritoneal mesotheliomas. The diagnosis of malignant mesothelioma was based upon the characteristic clinicoradiographic and histopathological features. Occupational asbestos exposure was documented in the vast majority of cases. The tissue specimens were mainly derived from post-mortem examination (77% autopsy, 23% biopsy) and had uniformly been formalin-fixed and paraffin-embedded. They had all been originally examined and classified by one experienced lung pathologist (PV) and were reviewed (MH) to identify suitable areas for tissue microarray construction. Any discrepancy between the current assessment and original diagnosis was resolved by consensus upon simultaneous review (MH, PV and HM).
Tissue Microarray Construction
The construction of a set of three tissue microarrays was accomplished with a custom-made, semiautomatic tissue arrayer (Beecher Instruments, Sun Prairie, WI, USA) as described before.40, 46 The available tumors had been reinvestigated and grouped into three categories: epithelioid, sarcomatoid and biphasic malignant mesothelioma. Distinct areas with different morphological growth patterns had been marked. The most representative tumor blocks were then selected and four tissue cores, 0.6 mm in diameter, were taken from each case and transferred into the recipient paraffin block. Wherever applicable in biphasic mesotheliomas, areas were identified that clearly separated the two different growth patterns, epithelioid and sarcomatoid, and two cores were taken from each area, enabling us to compare directly the phenotypic diversity in one and the same tumor.
Immunohistochemistry
Sections (4.5 μm) of tissue microarray blocks were transferred to an adhesive-coated slide system (Instrumedics, Hackensack, NJ, USA) supporting the cohesion of 0.6 mm array elements on glass. De-paraffined sections were manually stained after heat-induced epitope retrieval (3 min, 110°C, citrate buffer, pH 6.0) using a standard multilink detection kit (Medi-Stain HRP DAB, mediteAG, Nunningen, CH, Switzerland), including endogenous peroxidase block, block of nonspecific binding, horse radish peroxidase, and diaminobenzidene as chromogen. The primary antibodies were a polyclonal rabbit antiserum for calretinin (Cell Marque, Hot Springs, AR, USA), prediluted by the manufacturer, and a monoclonal mouse antibody for podoplanin (clone D2-40) (DakoCytomation, Baar, CH, Switzerland) at 1:50 dilution. They were used for overnight incubation at 4°C. For both antibodies adequate positive controls were used, vermiform appendix for calretinin and uterus for D2-40, according to the manufacturer's recommendations.
Interpretation of Results
The sections were semiquantitatively assessed by one observer (MH) and assistance was received for ambiguous cases (HM, PV). The growth pattern was confirmed on every individual spot. If both growth patterns were present on one spot of a biphasic malignant mesothelioma we rated them independently of each other, if the number of cells attributed to each type allowed us to do so. In a first step, staining intensity of positive tumor cells was recorded as follows: weak (1+), moderate (2+) or strong (3+). Strong staining intensity was defined as staining comparable to control tissue. Weak staining was regarded as any faint staining in tumor cells.
In a second step, the percentage of positive tumor cells was calculated and a three-tiered score (negative, weak and strong staining) was generated for statistical analysis. All tumors without staining or staining in up to 10% of tumor cells were regarded as negative. Tumors with 1+ staining intensity in more than 10% or 2+ intensity in 10–30% of cells were considered weakly positive. Spots with 2+ intensity in 30% tumor cells and more or 3+ intensity in more than 10% of cells were considered strongly positive. The relatively high cutoff value of 10% was set to rule out false positive results.
Fluorescence In Situ Hybridization
For FISH, a Dual Color Break Apart Rearrangement Probe for the detection of SYT (18q 11.2) gene translocation (LSI® SYT (18q 11.2), Abbott/Vysis Molecular Diagnostics, IL, USA) was used according to the manufacturer's instructions with minor modifications. These included tissue preincubation with 30 mg pepsin (Sigma) at 37°C for 25 min. FISH signals of at least 40 nuclei were evaluated for each spot (four spots per case), using a fluorescence microscope (Olympus BX61) with filters DAPI, Spectrum Orange and Spectrum Green. The images were acquired with a CCD camera and analyzed with AnalySIS software (Soft Imagining System, Munster, Germany). If at least two spots of each case contained not less than 25% of split red and green signals, the tumor was regarded as being positive for the SYT translocation.52 The signal was considered as split if the red and green signals were smallest separated by more than twice the distance occupied by a several signal.
Statistics
All cases were morphologically and/or immunologically defined mesotheliomas. Therefore, the sensitivity for D2-40 and calretinin could be calculated with the formula: Sensitivity=true positives/(true positives+false negatives).
Results
Tumors
The tissue microarray comprised a total of 1372 tissue cylinders. For calretinin, 816 epithelioid and 369 sarcomatoid spots, and 139 with both growth patterns could be evaluated. For D2-40, there were 818 epithelioid and 373 sarcomatoid spots, and 140 with both growth patterns. Forty-eight spots for calretinin, and 41 spots for D2-40 were not interpretable because of necrosis or lack of sufficient cells on the spot. The average of evaluable cells per spot was 340 tumor cells (minimum 80 tumor cells). Two cases were lost entirely on both slides because none of the four spots was interpretable. After exclusion of these tumors, 341 malignant mesotheliomas remained for statistical analysis.
Calretinin
There was both, cytoplasmic and nuclear staining for calretinin (Figure 1a). Two hundred fifty-two (91%) epithelioid malignant mesotheliomas or biphasic malignant mesotheliomas with an epithelioid component were calretinin positive. Twenty-two (8%) cases showed weak and 230 (83%) strong positivity. In contrast, only 95 (57%) sarcomatoid malignant mesotheliomas or biphasic malignant mesotheliomas with a sarcomatoid component exhibited calretinin positivity. Thirty-four (20%) showed weak and 61 (37%) strong calretinin positivity (Table 1).
D2-40
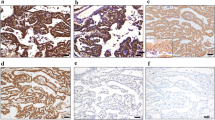
Podoplanin expression was seen in 180 (66%) epithelioid malignant mesotheliomas or epithelioid components of biphasic malignant mesotheliomas (6% weak and 60% strong positivity). Fifty (30%) sarcomatoid malignant mesotheliomas or sarcomatoid components of biphasic malignant mesotheliomas were D2-40 positive (6% weak and 24% strong positivity). D2-40 positive tumor cells showed membranous and/or cytoplasmic staining. Immunostaining was frequently patchy. Epithelioid areas showed predominantly membranous D2-40 staining whereas sarcomatoid areas exhibited cytoplasmic staining (Figure 1b and c; Tables 2 and 3). An interesting observation was a dot-like D2-40 staining in a few cells, which was detected in five cases (Figure 1d).
Sensitivity of Calretinin and D2-40
There was no significant difference, neither for calretinin nor for D2-40 staining, between tissue from pure epithelioid and sarcomatoid malignant mesothelioma compared to epithelioid and sarcomatoid areas of biphasic malignant mesothelioma. Thus, sensitivity was calculated separately in epithelioid and sarcomatoid differentiated areas, independent of its original type. Calretinin reached a sensitivity of 0.91 for epithelioid and 0.57 for sarcomatoid areas. The sensitivity of D2-40 was 0.66 and 0.3, respectively (Table 4). A combination of D2-40 and calretinin increased the sensitivity to detect sarcomatoid malignant mesothelioma to 0.66 and epithelioid malignant mesothelioma to 0.96. The percentage of additional malignant mesotheliomas detected using a panel of calretinin and D2-40 was 8.5% for sarcomatoid malignant mesothelioma and 4.5% for epithelioid malignant mesothelioma (Figure 2). There was one desmoplastic variant of sarcomatoid mesothelioma, which was negative for calretinin and D2-40. There were no tumors with heterologous differentiation.
Calretinin (blue) and D2-40 (red) positivity in epithelioid (a) and sarcomatoid (b) tumor areas of malignant mesothelioma. (a) Thirty percent of epithelioid areas were positive only for calretinin, 4% only for D2-40 and 61% for both. No expression for both markers in 4% of epithelioid areas (blank). (b) Thirty-six percent of sarcomatoid areas were positive only for calretinin, 8% only for D2-40 and 22% for both. No expression for both markers in 34% of sarcomatoid areas.
Discussion
In this study, we have used the tissue microarray technology to compare two mesothelioma antibodies, calretinin and D2-40. The use of tissue microarrays allowed to compare the sensitivity of both antibodies within epithelioid and sarcomatoid components of malignant mesothelioma. We have shown that calretinin is of superior value in detecting both epithelioid and sarcomatoid components of malignant mesothelioma.
Numerous investigations have meanwhile provided strong evidence on the representativeness of tissue microarray data for biomarker analysis. Tissue microarray studies have almost always reproduced the expected biological relevance of a given biomarker, for example, the clinical significance of Ki-67 labeling index in bladder cancer, the prognostic role of steroid hormone receptor expression and Her2 neu amplification/overexpression in breast cancer as well as many other established or new associations between molecular markers and tumor phenotype or clinical outcome.41, 45, 48 Also in our study, there was a high concordance of tissue microarray results and data obtained on large tissue specimens. Previous studies have found calretinin positivity in 50–100% of epithelioid malignant mesothelioma, with only very few studies showing expression frequencies below 90% on large tissue sections. In our large tissue microarray analysis, we observed calretinin expression in 91% of epithelioid areas of malignant mesothelioma. A previous tissue microarray analysis reported calretinin positivity in 85.7%.22 Therefore, the present study is another example for the representativeness of tissue microarrays.
In contrast to the traditional large section method, tissue microarrays are an ideal tool to compare different antibodies. Large section studies generate a huge workload and the number of tumors always remains low if multiple parameters are analyzed. Recent large section studies examining diagnostic antibody panels in malignant mesotheliomas only included 173, 111 or 40 malignant mesotheliomas.24, 53, 54 It is evident that the use of larger mesothelioma numbers has a higher statistical value for the evaluation of diagnostic markers. Since other proposed diagnostic markers can easily be included in one study, the use of tissue microarrays allows to rapidly determine optimal antibody panels for diagnostic purposes and to accelerate characterization of novel markers.
The availability of mesothelioma-specific antibodies facilitated the differential diagnosis between adenocarcinoma and epithelioid malignant mesothelioma. However, the diagnostic spectrum of spindle cell lesions of the pleura has also been expanded by other entities, recently. It includes pleural synovial sarcomas, pulmonary sarcomatoid carcinomas, malignant solitary fibrous tumor, malignant pleural smooth muscle tumor and extraskeletal osteosarcoma.55 Therefore, exact knowledge of the sensitivity of malignant mesothelioma markers is of increasing importance. To obtain a comprehensive overview of two different diagnostic antibodies in different tumor components, we have selected tissue cores from both epithelial and sarcomatoid areas. By this approach, we could immediately compare the sensitivity of both antibodies in different histologic types of malignant mesothelioma.
Noteworthy, there are only limited data on calretinin expression in sarcomatoid differentiated areas of malignant mesothelioma in the literature and the results diverge from 18 to 100% positivity in sarcomatoid malignant mesothelioma. We found calretinin staining in 57% and D2-40 staining in only 30% of areas with sarcomatoid differentiation. These results are consistent with data by Chu et al23 but contrast with data by Ordóñez,24 who was not able to detect D2-40 immunoreactivity in sarcomatoid malignant mesothelioma. Importantly, the sensitivity of D2-40 was lower than that of calretinin in both, epithelioid and sarcomatoid areas. We expected a higher sensitivity for D2-40 than calretinin in sarcomatoid malignant mesothelioma, because D2-40 is also positive in other spindle cell lesions, for example Kaposi's sarcoma, and lymphangioma, whereas calretinin is expressed in many epithelioid tumors of non-mesothelial origin, for example, Merkel cell carcinoma, Leydig cell tumors of the testis, esthesioneuroblastoma, adenomas of the adrenal gland and small cell carcinoma of the lung.5, 21, 22 According to our data, calretinin is a more robust marker for malignant mesothelioma than D2-40, although D2-40 probably does increase the specificity of an immunohistochemical panel considerably, because calretinin staining by itself can be seen in a variety of tumors. A panel including calretinin and D2-40 would increase the sensitivity especially for the detection of sarcomatoid malignant mesothelioma.
In order to rule out the presence of synovial sarcomas in our mesothelioma specific tissue microarray, we performed a fluorescence in situ hybridization for detection of the translocation t(X,18). Synovial sarcomas have previously been found to be reactive for D2-4024 and calretinin.15, 56, 57, 58 Unfortunately, we were able to obtain sufficient FISH signals in only 18% of the tumors. This low number might be due to different fixation procedures used in different institutions, but the presence of synovial sarcomas could be ruled out at least in these cases (Figure 3).
In summary, our results demonstrate that calretinin is of superior diagnostic value than D2-40. A diagnostic antibody panel including calretinin and D2-40 significantly improves the detection rate of sarcomatoid malignant mesothelioma.
References
Moch H, Oberholzer M, Dalquen P, et al. Diagnostic tools for differentiating between pleural mesothelioma and lung adenocarcinoma in paraffin embedded tissue. Part I: Immunohistochemical findings. Virchows Arch A Pathol Anat Histopathol 1993;423:19–27.
Moch H, Oberholzer M, Christen H, et al. Diagnostic tools for differentiating pleural mesothelioma from lung adenocarcinoma in paraffin embedded tissue. II. Design of an expert system and its application to the diagnosis of mesothelioma. Virchows Arch A Pathol Anat Histopathol 1993;423:493–496.
Ordonez NG . The immunohistochemical diagnosis of mesothelioma: a comparative study of epithelioid mesothelioma and lung adenocarcinoma. Am J Surg Pathol 2003;27:1031–1051.
Gotzos V, Vogt P, Celio MR . The calcium binding protein calretinin is a selective marker for malignant pleural mesotheliomas of the epithelial type. Pathol Res Pract 1996;192:137–147.
Doglioni C, Tos AP, Laurino L, et al. Calretinin: a novel immunocytochemical marker for mesothelioma. Am J Surg Pathol 1996;20:1037–1046.
Riera JR, Astengo-Osuna C, Longmate JA, et al. The immunohistochemical diagnostic panel for epithelial mesothelioma: a reevaluation after heat-induced epitope retrieval. Am J Surg Pathol 1997;21:1409–1419.
Leers MP, Aarts MM, Theunissen PH . E-cadherin and calretinin: a useful combination of immunochemical markers for differentiation between mesothelioma and metastatic adenocarcinoma. Histopathology 1998;32:209–216.
Ordonez NG . Value of calretinin immunostaining in differentiating epithelial mesothelioma from lung adenocarcinoma. Mod Pathol 1998;11:929–933.
Chenard-Neu MP, Kabou A, Mechine A, et al. Immunohistochemistry in the differential diagnosis of mesothelioma and adenocarcinoma. Evaluation of 5 new antibodies and 6 traditional antibodies. Ann Pathol 1998;18:460–465.
Cury PM, Butcher DN, Fisher C, et al. Value of the mesothelium-associated antibodies thrombomodulin, cytokeratin 5/6, calretinin, and CD44H in distinguishing epithelioid pleural mesothelioma from adenocarcinoma metastatic to the pleura. Mod Pathol 2000;13:107–112.
Oates J, Edwards C . HBME-1, MOC-31, WT1 and calretinin: an assessment of recently described markers for mesothelioma and adenocarcinoma. Histopathology 2000;36:341–347.
Brockstedt U, Gulyas M, Dobra K, et al. An optimized battery of eight antibodies that can distinguish most cases of epithelial mesothelioma from adenocarcinoma. Am J Clin Pathol 2000;114:203–209.
Carella R, Deleonardi G, D'Errico A, et al. Immunohistochemical panels for differentiating epithelial malignant mesothelioma from lung adenocarcinoma: a study with logistic regression analysis. Am J Surg Pathol 2001;25:43–50.
Comin CE, Novelli L, Boddi V, et al. Calretinin, thrombomodulin, CEA, and CD15: a useful combination of immunohistochemical markers for differentiating pleural epithelial mesothelioma from peripheral pulmonary adenocarcinoma. Hum Pathol 2001;32:529–536.
Miettinen M, Limon J, Niezabitowski A, et al. Calretinin and other mesothelioma markers in synovial sarcoma: analysis of antigenic similarities and differences with malignant mesothelioma. Am J Surg Pathol 2001;25:610–617.
Roberts F, McCall AE, Burnett RA . Malignant mesothelioma: a comparison of biopsy and postmortem material by light microscopy and immunohistochemistry. J Clin Pathol 2001;54:766–770.
Tot T . The value of cytokeratins 20 and 7 in discriminating metastatic adenocarcinomas from pleural mesotheliomas. Cancer 2001;92:2727–2732.
Abutaily AS, Addis BJ, Roche WR . Immunohistochemistry in the distinction between malignant mesothelioma and pulmonary adenocarcinoma: a critical evaluation of new antibodies. J Clin Pathol 2002;55:662–668.
Lucas DR, Pass HI, Madan SK, et al. Sarcomatoid mesothelioma and its histological mimics: a comparative immunohistochemical study. Histopathology 2003;42:270–279.
Ordonez NG . The diagnostic utility of immunohistochemistry in distinguishing between mesothelioma and renal cell carcinoma: a comparative study. Hum Pathol 2004;35:697–710.
Tos AP, Doglioni C . Calretinin: a novel tool for diagnostic immunohistochemistry. Adv Anat Pathol 1998;5:61–66.
Lugli A, Forster Y, Haas P, et al. Calretinin expression in human normal and neoplastic tissues: a tissue microarray analysis on 5233 tissue samples. Hum Pathol 2003;34:994–1000.
Chu AY, Litzky LA, Pasha TL, et al. Utility of D2-40, a novel mesothelial marker, in the diagnosis of malignant mesothelioma. Mod Pathol 2005;18:105–110.
Ordonez NG . D2-40 and podoplanin are highly specific and sensitive immunohistochemical markers of epithelioid malignant mesothelioma. Hum Pathol 2005;36:372–380.
Marks A, Sutherland DR, Bailey D, et al. Characterization and distribution of an oncofetal antigen (M2A antigen) expressed on testicular germ cell tumours. Br J Cancer 1999;80:569–578.
Schacht V, Dadras SS, Johnson LA, et al. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am J Pathol 2005;166:913–921.
Ma T, Yang B, Matthay MA, et al. Evidence against a role of mouse, rat, and two cloned human t1alpha isoforms as a water channel or a regulator of aquaporin-type water channels. Am J Respir Cell Mol Biol 1998;19:143–149.
Martin-Villar E, Scholl FG, Gamallo C, et al. Characterization of human PA2.26 antigen (T1alpha-2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas. Int J Cancer 2005;113:899–910.
Kahn HJ, Bailey D, Marks A . Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol 2002;15:434–440.
Fukunaga M . Expression of D2-40 in lymphatic endothelium of normal tissues and in vascular tumours. Histopathology 2005;46:396–402.
Amin KM, Litzky LA, Smythe WR, et al. Wilms' tumor 1 susceptibility (WT1) gene products are selectively expressed in malignant mesothelioma. Am J Pathol 1995;146:344–356.
Clover J, Oates J, Edwards C . Anti-cytokeratin 5/6: a positive marker for epithelioid mesothelioma. Histopathology 1997;31:140–143.
Roberts F, Harper CM, Downie I, et al. Immunohistochemical analysis still has a limited role in the diagnosis of malignant mesothelioma. A study of thirteen antibodies. Am J Clin Pathol 2001;116:253–262.
Miettinen M, Sarlomo-Rikala M . Expression of calretinin, thrombomodulin, keratin 5, and mesothelin in lung carcinomas of different types: an immunohistochemical analysis of 596 tumors in comparison with epithelioid mesotheliomas of the pleura. Am J Surg Pathol 2003;27:150–158.
Hirano H, Kizaki T, Sashikata T, et al. Synovial sarcoma arising from the pleura: a case report with ultrastructural and immunohistological studies. Med Electron Microsc 2002;35:102–108.
Colwell AS, D'Cunha J, Vargas SO, et al. Synovial sarcoma of the pleura: a clinical and pathologic study of three cases. J Thorac Cardiovasc Surg 2002;124:828–832.
Carbone M, Rizzo P, Powers A, et al. Molecular analyses, morphology and immunohistochemistry together differentiate pleural synovial sarcomas from mesotheliomas: clinical implications. Anticancer Res 2002;22:3443–3448.
Ng SB, Ahmed Q, Tien SL, et al. Primary pleural synovial sarcoma. A case report and review of the literature. Arch Pathol Lab Med 2003;127:85–90.
Vohra HA, Davies S, Vohra H, et al. Primary synovial sarcoma of the pleura: beware of misdiagnosis. Eur J Intern Med 2004;15:465–466.
Kononen J, Bubendorf L, Kallioniemi A, et al. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med 1998;4:844–847.
Schraml P, Kononen J, Bubendorf L, et al. Tissue microarrays for gene amplification surveys in many different tumor types. Clin Cancer Res 1999;5:1966–1975.
Camp RL, Charette LA, Rimm DL . Validation of tissue microarray technology in breast carcinoma. Lab Invest 2000;80:1943–1949.
Moch H, Kononen T, Kallioniemi OP, et al. Tissue microarrays: what will they bring to molecular and anatomic pathology? Adv Anat Pathol 2001;8:14–20.
Hoos A, Urist MJ, Stojadinovic A, et al. Validation of tissue microarrays for immunohistochemical profiling of cancer specimens using the example of human fibroblastic tumors. Am J Pathol 2001;158:1245–1251.
Nocito A, Bubendorf L, Maria Tinner E, et al. Microarrays of bladder cancer tissue are highly representative of proliferation index and histological grade. J Pathol 2001;194:349–357.
Bubendorf L, Nocito A, Moch H, et al. Tissue microarray (TMA) technology: miniaturized pathology archives for high-throughput in situ studies. J Pathol 2001;195:72–79.
Nocito A, Kononen J, Kallioniemi OP, et al. Tissue microarrays (TMAs) for high-throughput molecular pathology research. Int J Cancer 2001;94:1–5.
Torhorst J, Bucher C, Kononen J, et al. Tissue microarrays for rapid linking of molecular changes to clinical endpoints. Am J Pathol 2001;159:2249–2256.
Gulmann C, Butler D, Kay E, et al. Biopsy of a biopsy: validation of immunoprofiling in gastric cancer biopsy tissue microarrays. Histopathology 2003;42:70–76.
Jourdan F, Sebbagh N, Comperat E, et al. Tissue microarray technology: validation in colorectal carcinoma and analysis of p53, hMLH1, and hMSH2 immunohistochemical expression. Virchows Arch 2003;443:115–121.
Rosen DG, Huang X, Deavers MT, et al. Validation of tissue microarray technology in ovarian carcinoma. Mod Pathol 2004;17:790–797.
Bode B, Frigerio S, Behnke S, et al. Mutations in the tyrosine kinase domain of the EGFR gene are rare in synovial sarcoma. Mod Pathol 2006;19:541–547.
Granville LA, Younes M, Churg A, et al. Comparison of monoclonal versus polyclonal calretinin antibodies for immunohistochemical diagnosis of malignant mesothelioma. Appl Immunohistochem Mol Morphol 2005;13:75–79.
Soomro IN, Oliveira R, Ronan J, et al. Expression of mesothelial markers in malignant mesotheliomas: an immunohistochemical evaluation of 173 cases. J Pak Med Assoc 2005;55:205–209.
Knuuttila A, Jee KJ, Taskinen E, et al. Spindle cell tumours of the pleura: a clinical, histological and comparative genomic hybridization analysis of 14 cases. Virchows Arch 2006;448:135–141.
Cappello F, Barnes L . Synovial sarcoma and malignant mesothelioma of the pleura: review, differential diagnosis and possible role of apoptosis. Pathology 2001;33:142–148.
Cappello F, Bellafiore M, Bucchieri F, et al. Poorly differentiated synovial sarcoma: a case report. Pathol Oncol Res 2001;7:63–66.
Mikami Y, Nakajima M, Hashimoto H, et al. Primary poorly differentiated monophasic synovial sarcoma of the lung. A case report with immunohistochemical and genetic studies. Pathol Res Pract 2003;199:827–833; discussion 835–836.
Acknowledgements
We thank for the support of Ms P Wälchli (Pneumoconiosis Research Group Zurich) and Mrs S Behnke for technical support in immunohistochemistry. This study was partly supported by a grant from UBS AG Switzerland (made possible by an anonymous donor).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hinterberger, M., Reineke, T., Storz, M. et al. D2-40 and calretinin—a tissue microarray analysis of 341 malignant mesotheliomas with emphasis on sarcomatoid differentiation. Mod Pathol 20, 248–255 (2007). https://doi.org/10.1038/modpathol.3800736
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800736
Keywords
This article is cited by
-
GATA3 is a useful immunohistochemical marker for distinguishing sarcomatoid malignant mesothelioma from lung sarcomatoid carcinoma and organizing pleuritis
Virchows Archiv (2021)
-
Podoplanin is a useful prognostic marker and indicates better differentiation in lung squamous cell cancer patients? A systematic review and meta-analysis
BMC Cancer (2020)
-
Diagnostic Accuracy of Calretinin for Malignant Mesothelioma in Serous Effusions: a Meta-analysis
Scientific Reports (2015)
-
CD74: a new prognostic factor for patients with malignant pleural mesothelioma
British Journal of Cancer (2014)