Abstract
BRAF mutations are common events in a variety of melanocytic nevi and primary cutaneous melanomas. We have previously found BRAF mutations in 82% of nevi, consisting of congenital, common acquired and dysplastic types, and 33% of primary cutaneous melanomas other than the spitzoid type, similar to other published reports. A small number of studies have evaluated Spitz nevi and have failed to detect any lesions possessing a BRAF mutation. Only one study included categories of atypical Spitz nevus and borderline lesions suspected to be spitzoid melanomas, along with classic Spitz nevi and spitzoid melanomas. We examined a spectrum of spitzoid lesions that included 48 Spitz nevi, some with atypical features, seven atypical (borderline) Spitz tumors, and 13 spitzoid melanomas. BRAF mutations were detected in 12 of 68 spitzoid lesions, of which two were spitzoid melanomas and 10 were Spitz nevi. Five of the 10 Spitz nevi with BRAF mutations were altered by more than usual cytologic atypia and/or architectural atypia overlapping with dysplastic nevi, or irritation/inflammation; one desmoplastic Spitz nevus had a BRAF mutation. These results indicate that a small subset of Spitz nevi, some with atypical histologic features, possess BRAF mutations. Therefore, the BRAF mutational status does not separate all Spitz nevi from spitzoid melanomas and non-Spitz types of melanocytic proliferations, contrary to previous reports.
Similar content being viewed by others
Main
In 1948, Sophie Spitz described a series of 13 patients with ‘melanomas in childhood’, one of whom died from dissemination of metastatic melanoma. She considered these lesions to be juvenile melanomas with a generally better prognosis than conventional melanomas in adulthood.1 Shortly thereafter, it was asserted that these lesions were, in fact, benign nevi because of their indolent behavior.2, 3 Spitz's seminal paper underscored the difficulties in distinguishing some Spitz nevi from melanomas with Spitz-like features that can metastasize and potentially eventuate in a fatal outcome. While criteria have been published over the years in an effort to distinguish Spitz nevi from Spitz-like melanomas, this diagnostic dilemma continues to plague dermatopathologists. It is readily apparent that there may be a lack of consensus in the diagnosis of spitzoid lesions even among experts in this field.3, 4
Fortunately, most spitzoid lesions can be classified into benign Spitz nevi or Spitz-like melanomas based on published criteria.5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 However, a subset of spitzoid lesions remain that have histologic features that deviate from a typical Spitz nevus, yet are insufficient for a definitive diagnosis of Spitz-like melanoma. These atypical spitzoid lesions have been referred to variously in the literature as borderline and intermediate melanocytic neoplasia, minimal-deviation melanoma, nevoid melanoma, atypical Spitz nevus/tumor, malignant Spitz nevus, problematic Spitzoid melanocytic lesions, and diagnostically controversial Spitzoid melanocytic tumors.16, 17, 18, 19, 20, 21, 22
Recently, interest in the RAS-RAF-MEK-ERK-MAP kinase signal transduction pathway has arisen as a site for mutational analysis in a broad spectrum of melanocytic lesions, including Spitz nevi. In a landmark study by Davies et al,23 66% of malignant melanomas were shown to harbor a T1796A BRAF mutation in exon 15 resulting in the substitution of valine by glutamic acid at position 600 (V600E). Subsequently, Pollock et al24 demonstrated a high incidence (82%) of the same BRAF mutation in a variety of non-Spitz nevi, similar to their primary invasive melanoma group. Since then, many studies have reported BRAF mutations in benign and malignant melanocytic lesions.25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39 However, a number of studies have failed to demonstrate BRAF mutations in Spitz nevi.25, 40, 41, 42, 43, 44, 45, 46, 57, 58, 59, 60, 61 Most of these studies analyzed only classic Spitz nevi. Only one study included atypical Spitz nevi and histologically borderline spitzoid lesions in their BRAF analysis.40
The purpose of this study was to evaluate BRAF and NRAS mutations in a spectrum of spitzoid lesions, which included classic and atypical Spitz nevi, atypical Spitz tumors of uncertain biologic behavior and spitzoid melanomas, to determine whether the presence or absence of these mutations can distinguish among the different groups of spitzoid lesions and to compare our results to existing data in the literature.
Materials and methods
Case Selection
A spectrum of 68 spitzoid melanocytic lesions, including 48 Spitz nevi, seven atypical Spitz tumors, and 13 spitzoid melanomas, were retrieved from the archives of the Pathology Department at the University of Michigan. The spitzoid lesions were independently reviewed by three board certified dermatopathologists (LL, LDS, and DRF) with diagnostic expertise in pigmented lesions and who are members of the Multidisciplinary Melanoma Clinic at the University of Michigan. Of the 48 Spitz nevi, 21 were classic Spitz nevi, on the basis of previously published criteria,6, 7 and two were desmoplastic Spitz nevi. The remaining 25 Spitz nevi demonstrated some atypical features, such as architectural disorder, increased cytologic atypia and/or inflammation. This subset of Spitz nevus has been referred to in the literature as atypical or dysplastic Spitz nevus.47 Nonetheless, as these atypical lesions still retain the salient histologic criteria for Spitz nevi, they are classified within the Spitz nevus group. The seven lesions classified as atypical Spitz tumors shared some histologic criteria with conventional Spitz nevi, such as small diameter, symmetry, lateral circumscription, epidermal hyperplasia, and/or Kamino bodies. These lesions, however, showed histologic features that significantly deviated from conventional Spitz nevus, yet lacked sufficient histologic criteria for Spitz-like melanoma. These criteria included expansile or sheet-like dermal growth, incomplete to absent dermal maturation, bulbous extension into the deep dermis or subcutis, deep dermal mitoses, and/or high-grade nuclear atypia.10, 13, 16, 21, 22, 48 Although the 13 spitzoid melanomas maintained some low-power resemblance to Spitz nevi, they demonstrated at least one, and often multiple, of the following histologic features: asymmetrical growth, lack of lateral circumscription, pagetoid spread of melanocytes in the epidermis, aberrant dermal growth, dermal mitoses at all levels of the lesion, atypical mitoses, and high-grade nuclear atypia.9, 10, 12, 13, 48 The Institutional Review Board at the University of Michigan has approved this study.
DNA Extraction
DNA was extracted from slides as previously described.49 Briefly, lesional DNA was microdissected from unstained slides from paraffin-embedded tissue blocks. Areas of microdissection were circled on corresponding H&E stained slides by one dermatopathologist (DRF), which in turn were used as a template. Following dissection from the slides, xylene was added to remove paraffin and the DNA was precipitated with ethanol. Following centrifugation, the supernatant was discarded and the pellet was lyophilized. The pellet was resuspended in 100 μl Proteinase K buffer (50 mM tris and 200 ng/μl Proteinase K). The samples were incubated overnight at 37°C and then denatured at 95°C.
Mutational Analysis
Mutations in BRAF codon 600 were identified by direct sequencing of exon 15 of BRAF following PCR amplification of DNA extracted from paraffin-embedded samples. NRAS mutations were identified by direct sequencing of exons 1 and 2 of NRAS following PCR amplification. PCR reactions included 10 mM Tris-HCl, pH 8.3, 50 mM KCl, 1.5 mM MgCl2, 200 μM dNTPs, 100 ng both forward and reverse primer, 1.5 U AmpliTaq Gold (Applied Biosystems), and 2 μl microdissected tumor DNA in a total volume of 50 μl. Samples were denatured for 5 min at 95°C and were passed through 40 cycles of amplification, which consisted of 60 s of denaturation at 95°C, 1 min of primer annealing at 56°C, and 1 min of elongation at 72°C. The DNA sequences of the primers for exon 15 of the BRAF gene were forward: 5′TCATAATGCTTGCTCTGATAGGA and reverse: 5′GGCCAAAAATTTAATCAGTGGA.23 The DNA sequences of the primers for exon 1 of the NRAS gene were either forward: 5′ATGACTGAGTACAAACTGGT and reverse: 5′CTCTATGGTGGGTCATATT or forward: 5′ATGACTGAGTACAAACTGGT and reverse: 5′CTCTATGGTGGGATCATATT. The sequences of the primers for exon 2 of the NRAS gene were forward: 5′GGTGAAACCTGTTTGTTGGA and reverse: 5′ATACACAGAGGAAGCCTTC. All sequencing for BRAF and NRAS mutations was performed on the ABI 3700 automated DNA sequencer (Applied Biosystems, Foster City, CA, USA). BRAF mutations were detected by using Mutation Surveyor™ software (Softgenetics Inc., State College, PA, USA), and confirmed by visual inspection of chromatograms by two independent readers (JNP, SBG).
Statistical Methods
All statistical analyses were performed using SASv.9.1 for Windows (SAS Institute, Cary, NC, USA). Fisher's Exact test was used to test for differences in BRAF frequencies between groups. The Wilcoxon rank sum test was used to test the difference in median age by mutation status.
Results
The clinical features and BRAF status of spitzoid lesions from 68 patients are summarized in Table 1. Spitz nevi were removed from 24 males and 24 females and the patients ranged from 2 to 49 years (median=19 years) in age. Spitz nevi were located on the head and neck (10/48, 21%), trunk (9/48, 19%), or extremities (26/48, 54%); the anatomic location was not specified for three (6%) Spitz nevi.
Atypical Spitz tumors were more common in females5 than males,2 and patients ranged in age from 12 to 52 years (median=24 years). Atypical Spitz tumors were located on the head and neck (1/7, 14%), trunk (1/7, 14%), and extremities (5/7, 72%). All seven atypical Spitz tumors had sentinel lymph node biopsies that were negative for metastatic disease.
Spitzoid melanomas were diagnosed in 10 female and three male patients. The patients ranged in age from 10 to 60 years old (median=24 years). Spitzoid melanomas were removed from the head and neck (1/13, 8%), trunk (2/13, 15%), and extremities (10/13, 77%). Five of 10 (50%) spitzoid melanomas had positive sentinel lymph nodes for metastatic melanoma. Sentinel lymph node biopsy was not performed in three patients with thin (<1 mm) spitzoid melanomas.
In 12 of 68 (18%) patients, a full set of diagnostic slides were not available for review. The initial diagnosis was rendered by one of the three dermatopathologists in this study but the slides were returned to the referring institution and were no longer available for review by the other two dermatopathologists at the time of this study. The slides from the remaining 55 of 68 patients (81%) were independently reviewed by three dermatopathologists. There was concordance among all three dermatopathologists in classifying 41 of 55 (75%) lesions into one of the three groups of spitzoid lesions. There was, however, discordance among one of the three dermatopathologists in classifying 14 of 55 (25%) spitzoid lesions. In no instance did all three dermatopathologists completely disagree in the classification of a spitzoid lesion across all three categories.
The diagnosis of record for cases where there was diagnostic discordance in this study was the diagnosis rendered by two of the three dermatopathologists. The most common cause for discordance among dermatopathologists was in classifying lesions between Spitz nevi and atypical Spitz tumors (12/14, 75%). There was discordance in classifying two lesions between atypical Spitz tumors and spitzoid melanomas, one of which had a sentinel lymph node biopsy performed that was negative for metastatic melanoma. The distinction between an atypical Spitz tumor and a spitzoid melanoma is a predictable dilemma and in our institution these lesions are treated similarly based on the maximum depth of dermal extension.
BRAF (V600E) mutations were detected in 12 of 68 (18%) spitzoid lesions (Figure 1). Ten of 12 (83%) spitzoid lesions with BRAF mutations were Spitz nevi, while the remaining two (17%) lesions were spitzoid melanomas. None of the seven atypical Spitz tumors had a BRAF mutation. There was no significant difference in the frequency of BRAF mutations by type of lesion (Exact P=0.60). Of the 10 Spitz nevi with BRAF mutations, five (50%) had histologic features of Spitz nevi and five (50%) had atypical histologic features, yet lacked histologic criteria of an atypical Spitz tumor or spitzoid melanoma (Figure 2). One Spitz nevus with a BRAF mutation was an intradermal desmoplastic Spitz nevus. Seven of the 10 Spitz nevi demonstrated some degree of epidermal hyperplasia and four cases had Kamino bodies. Five of these Spitz nevi were inflamed, at least focally, and two were interpreted as halo Spitz nevi. One Spitz nevus was irritated or traumatized, as evidenced by epidermal ulceration. Five atypical Spitz nevi had more than usual cytologic atypia and three also had some architectural disorder similar to an atypical (dysplastic) nevus. Of the 10 Spitz nevi with BRAF mutations, five occurred on the extremities, three on the trunk, and two on the head and neck. However, there was no significant difference in the proportion of all spitzoid lesions with BRAF mutations among the extremities (17%), trunk (25%) and head and neck (16%), (Exact P=0.89). The age of patients with BRAF mutations in Spitz nevi ranged from 2 to 38 (mean=16; median=14) years of age; five patients were less than 10 years old. There was no significant difference in the age of patients with Spitz nevi with BRAF status (mean=15.9 years) compared to those without BRAF mutations (mean=20.6 years), (P=0.32). Both spitzoid melanomas with BRAF mutations arose on the extremities, and the patients were 12 and 43 years of age (Table 2).
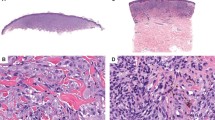
Chromatogram traces showing BRAF (V600E) mutations in (a) an atypical compound Spitz nevus (case 5; see Figures 2a and b for histopathology) and (b) a compound Spitz nevus (case 8; see Figures 2c and d for histopathology).
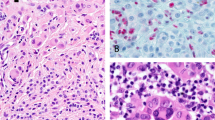
Histopathologic features of representative Spitz nevi with detectable BRAF mutations. Case 5 shows an atypical compound Spitz nevus with focal inflammation (a) and evidence of Kamino bodies within the epidermis (b). Case 8 demonstrates a compound Spitz nevus (c) with maturation of Spitz nevus cells with descent in the dermis (d). Case 2 shows a predominantly intradermal Spitz nevus with desmoplastic stromal response (e, f). Case 1 shows an irritated compound Spitz nevus with small nests of spitzoid melanocytes in the deepest portion of its dermal component (g, h). A spitzoid melanoma corresponding to case 12 has an aberrant dermal growth pattern of epithelioid spitzoid melanocytes with striking cytologic atypia (i).
Only one of 68 spitzoid lesions, an atypical Spitz nevus, had an NRAS mutation. No lesion demonstrated both NRAS and BRAF mutations.
Discussion
In a subset of spitzoid lesions, differentiating a Spitz nevus from a Spitz-like melanoma can be problematic and, subsequently, the confidence in rendering a definitive diagnosis declines. In such instances, attention has turned to ancillary techniques to aid in this distinction. Immunohistochemical studies have yielded variable results. For instance, stratification of HMB-45 has been demonstrated in Spitz nevi by some investigators but not by others.16, 18, 50, 51 Immunohistochemical staining with proliferation markers, such as Ki-67 and proliferating cell nuclear antigen, has yielded encouraging results in some studies.18, 51, 52, 53, 54, 55, 56, 57 The immunohistochemical pattern of S100A6 protein expression has been shown to significantly differ between Spitz nevi and other melanocytic nevi and melanomas.58 The mean silver staining pattern of the nucleolar organizer region (AgNOR) is lower in Spitz nevi compared to melanomas but there is overlap that limits its utility.59 Telomerase activity has been shown to be lowest in Spitz nevi by some groups,60, 61 whereas other investigators have found a similar telomerase activity in Spitz nevi when compared to ordinary nevi and melanomas.62
Molecular methods have been employed recently to evaluate a spectrum of melanocytic lesions, including Spitz nevi. Comparative genomic hybridization has shown chromosomal gains involving the p-arm of chromosome 11 and the q-arm of chromosome 7 in three of 17 and one of 17 Spitz nevi, respectively.63 Mutational analysis of the BRAF gene has shown that BRAF mutations are common events in most types of melanocytic nevi and melanomas. Similar to other investigators, we have previously shown that the highest incidence of BRAF mutations (82%) occurred in a group of congenital, common acquired, and atypical (dysplastic) nevi.24, 27, 42, 64 In contrast, 27% of primary invasive melanomas and 39% of metastatic melanomas had BRAF mutations in our series, which is similar to previously published data by other groups.25, 35, 39 The high incidence of BRAF mutations in nevi suggests that this is an early event in melanocytic neoplasia.
Several studies have evaluated the frequency of BRAF mutations in Spitz nevi and, to date, there have been no reported mutations in Spitz nevi.25, 40, 41, 42, 43, 44, 45, 46 With the exception of the study by van Dijk et al,40 which included an atypical Spitz nevus group, all other studies evaluated typical or classic Spitz nevi and/or spitzoid melanoma. The classic or typical Spitz nevus is usually readily distinguished from melanoma when standard histologic criteria are applied. However, when spitzoid lesions become increasingly atypical, the distinction of an atypical Spitz nevus/tumor from melanoma becomes more difficult and can be indistinguishable on histologic grounds in a small percentage of cases. Analyzing these atypical Spitz nevi and atypical Spitz tumors for BRAF mutations would be especially important to determine if their BRAF mutation status is more like that observed in classic Spitz nevi or non-Spitz nevi and melanomas.
In this study, we evaluated a spectrum of spitzoid lesions, which included typical and atypical Spitz nevi, atypical Spitz tumors of uncertain biologic potential, and spitzoid melanomas. In order to validate our classification of lesions, three dermatopathologists independently reviewed each spitzoid lesion based on previously published criteria. The concordance in the diagnosis of spitzoid lesions within our group was very good (75%), although there was discordance in 25% of the cases, which is to be expected based on previous reports in the literature.4, 5
In contrast to other series in the literature,25, 40, 41, 42, 43, 44, 45, 46 we identified BRAF mutations in 10 Spitz nevi, five of which specimens had atypical histologic features such as architectural disorder, increased cytologic atypia, and/or inflammation. The somewhat underpowered sample size of our study of the group of 48 Spitz nevi does not permit firm conclusions to be drawn with respect to age and BRAF status; however, we did not appreciate any statistically significant differences by age. We did note that the mean age of patients with BRAF-positive Spitz nevi (15.9 years) was slightly lower than the mean age of patients with BRAF-negative Spitz nevi (20.6 years), (P=0.32), which is consistent with observations in melanoma.65 Moreover, five of 10 Spitz nevi with BRAF mutations occurred in children less than 10 years old. The Spitz nevi possessing BRAF mutations were from a similar anatomic distribution as Spitz nevi without BRAF mutations in our study. We noted a slightly higher proportion of all spitzoid lesions on the trunk harboring BRAF mutations, but this was not statistically significant. The melanoma literature suggests that BRAF mutations are more common in melanomas arising in intermittently sun-exposed anatomic regions,65 but the power of the present study did not permit us to fully investigate this hypothesis for spitzoid lesions. It is not surprising that we found BRAF mutations in a small number of spitzoid melanomas, considering that spitzoid melanomas have been shown to have BRAF mutations in previous studies.40, 44
The novel finding in our study is the presence of BRAF mutations in a subset of Spitz nevi, which has not been previously reported. Most previous studies have limited their analysis to Spitz nevi with classic histologic features. The exception to this is the study by van Dijk et al,40 which included atypical Spitz nevi and spitzoid tumors suspected of being spitzoid melanomas along with their Spitz nevi and spitzoid melanoma groups. In contrast to our findings where we identified 10 (five atypical) Spitz nevi with BRAF mutations, they did not detect any BRAF mutations in their classic Spitz or atypical Spitz nevus groups. It is difficult to compare our findings to those of van Dijk et al, since they showed histologic images of a classic Spitz nevus, a spitzoid melanoma, and a lesion suspected of being a spitzoid melanoma but they did not show any histologic images of an atypical Spitz nevus. It is possible that different criteria were applied to these lesions between our and their groups of observers. Interestingly, one of our cases that possessed a BRAF mutation was an intradermal desmoplastic Spitz nevus. We had one other desmoplastic Spitz nevus in our series that lacked a BRAF mutation. Prior studies did not describe any lesions that were desmoplastic Spitz nevi, so there is no data available for comparison in the literature. Our low number of desmoplastic Spitz nevi precludes drawing any conclusion regarding BRAF status in this variant and requires accrual of additional cases for further evaluation.
Atypical Spitz tumors are problematic lesions because they possess overlapping histologic features between Spitz nevus and spitzoid melanoma. In our study, none of our atypical Spitz tumors had BRAF mutations, which is consistent with the results reported by van Dijk et al.40 This interpretation is limited, however, by the small sample size in this group. More cases of atypical Spitz tumors are required for analysis to add strength to this data.
NRAS mutations involving codon 61 and, to a lesser extent, codons 12 and 13, have been previously reported in melanomas and nevi occurring at sites of ultraviolet light exposure.26, 30, 32, 34, 36, 37, 38, 66, 67, 68, 69, 70, 71 Only one atypical Spitz nevus from the arm of a 22-year-old female had an NRAS mutation in our study. As expected, this lesion lacked a BRAF mutation, since NRAS and BRAF mutations are mutually exclusive events. An increased copy number of chromosome 11p by fluorescence in situ hybridization, corresponding to the HRAS gene, has been previously reported by Bastian et al.72 Subsequently, another group of investigators confirmed HRAS mutations in a low percentage of Spitz nevi and atypical Spitz nevi/tumors, but not in spitzoid melanomas, and did not identify any NRAS mutations.40 Other investigators, however, have failed to identify HRAS mutations in Spitz nevi or spitzoid melanomas.41 We did not perform HRAS mutational analysis in our spitzoid study group.
In conclusion, we report the presence of BRAF (V600E) mutations in a small subset of Spitz nevi, some demonstrating atypical histologic features, at a major melanoma referral center. This finding is contrary to previously published studies that have not detected BRAF mutations in any Spitz nevi to date. Thus, BRAF mutation status does not reliably distinguish all Spitz nevi from non-Spitz nevi and melanomas, as previously touted, and cannot be relied upon as a specific ancillary diagnostic tool in the evaluation of melanocytic lesions.
References
Spitz S . Melanomas of childhood. Am J Pathol 1948;24:591–609.
Allen AC . A reorientation of the histogenesis and clinical significance of cutaneous nevi and melanomas. Cancer 1949;2:28–55.
Allen AC, Spitz S . Malignant melanoma: a clinicopathological analysis of criteria for diagnosis and prognosis. Cancer 1953;6:1–45.
Farmer ER, Gonin R, Hanna MP . Discordance in the histopathologic diagnosis of melanoma and melanocytic nevi between expert pathologists. Hum Pathol 1996;27:528–531.
Barnhill RL, Argenyi ZB, From L, et al. Atypical Spitz nevi/tumors: lack of consensus for diagnosis, discrimination from melanoma, and prediction of outcome. Hum Pathol 1999;30:513–520.
Weedon D, Little JH . Spindle and epithelioid cell nevi in children and adults: a review of 211 cases of the Spitz nevus. Cancer 1977;40:217–225.
Paniago-Pereira C, Maize JC, Ackerman AB . Nevus of large spindle and/or epithelioid cells (Spitz's nevus). Arch Dermatol 1978;114:1811–1823.
Kamino H, Flotte TJ, Misheloff E, et al. Eosinophilic globules in Spitz's nevi. New findings and a diagnostic sign. Am J Dermatopathol 1979;1:319–324.
Crotty KA, McCarthy SW, Palmer AA, et al. Malignant melanoma in childhood: a clinicopathologic study of 13 cases and comparison with Spitz nevi. World J Surg 1992;16:179–185.
Barnhill RL, Flotte TJ, Fleischli M, et al. Cutaneous melanoma and atypical Spitz tumors in childhood. Cancer 1995;76:1833–1845.
Ackerman AB . Spitz nevus. Am J Dermatopathol 1997;19:419–421.
Walsh N, Crotty K, Palmer A, et al. Spitz nevi vs spitzoid malignant melanoma: an evaluation of the current distinguishing histopathologic criteria. Hum Pathol 1998;29:1105–1112.
Spatz A, Calonje E, Handfield-Jones S, et al. Spitz tumors in children: a grading system for risk stratification. Arch Dermatol 1999;135:282–285.
LeBoit P . Spitz nevus: a look back and a look ahead. Adv Dermatol 2000;16:81–109.
Mooi WJ . Histopathology of Spitz naevi and ‘Spitzoid’ melanomas. Curr Top Pathol 2001;94:65–77.
Smith KJ, Barrett TL, Skelton HG, et al. Spindle cell and epithelioid cell nevi with atypia and metastasis (malignant Spitz nevus). Am J Surg Pathol 1989;13:931–939.
Schmoeckel C, Castro CE, Braun-Falco O . Nevoid malignant melanoma. Arch Dermatol Res 1985;277:362–369.
McNutt NS, Urmacher C, Hakimian J, et al. Nevoid malignant melanoma: morphologic patterns and immunohistochemical reactivity. J Cutan Pathol 1995;22:502–517.
Reed RJ . Atypical Spitz nevus/tumor. Hum Pathol 1999;30:1523–1525.
Reed RJ . Dimensionalities: borderline and intermediate melanocytic neoplasia. Hum Pathol 1999;30:521–524.
Lohmann CM, Coit DG, Brady MS, et al. Sentinel lymph node biopsy in patients with diagnostically controversial spitzoid melanocytic tumors. Am J Surg Pathol 2002;26:47–55.
Su LD, Fullen DR, Sondak VK, et al. Sentinel lymph node biopsy in problematic spitzoid melanocytic lesions. Cancer 2003;97:499–507.
Davies H, Bignell GR, Cox C, et al. Mutations of the BRAF gene in human cancer. Nature 2002;417:949–954.
Pollock PM, Harper UL, Hansen KS, et al. High frequency of BRAF mutations in nevi. Nat Genet 2003;33:19–20.
Yazdi AS, Palmedo G, Flaig MJ, et al. Mutations of the BRAF gene in benign and malignant melanocytic lesions. J Invest Dermatol 2003;121:1160–1162.
Brose MS, Volpe P, Feldman M, et al. BRAF and RAS mutations in human lung cancer and melanoma. Cancer Res 2002;62:6997–7000.
Uribe P, Wistuba II, Gonzalez S . BRAF mutation: a frequent event in benign, atypical, and malignant melanocytic lesions of the skin. Am J Dermatopathol 2003;25:365–370.
Lang J, MacKie RM . Prevalence of exon 15 BRAF mutations in primary melanoma of the superficial spreading, nodular, acral, and lentigo maligna subtypes. J Invest Dermatol 2005;125:575–579.
Libra M, Malaponte G, Navolanic PM, et al. Analysis of BRAF mutation in primary and metastatic melanoma. Cell Cycle 2005;4:1382–1384.
Kumar R, Angelini S, Snellman E, et al. BRAF mutations are common somatic events in melanocytic nevi. J Invest Dermatol 2004;122:342–348.
Dong J, Phelps RG, Qiao R, et al. BRAF oncogenic mutations correlate with progression rather than initiation of human melanoma. Cancer Res 2003;63:3883–3885.
Goydos JS, Mann B, Kim HJ, et al. Detection of B-RAF and N-RAS mutations in human melanoma. J Am Coll Surg 2005;200:362–370.
Chang DZ, Panageas KS, Osman I, et al. Clinical significance of BRAF mutations in metastatic melanoma. J Transl Med 2004;2:46–50.
Pavey S, Johansson P, Packer L, et al. Microarray expression profiling in melanoma reveals a BRAF mutation signature. Oncogene 2004;23:4060–4067.
Shinozaki M, Fujimoto A, Morton DL, et al. Incidence of BRAF oncogene mutation and clinical relevance for primary cutaneous melanomas. Clin Cancer Res 2004;10:1753–1757.
Tsao H, Goel V, Wu H, et al. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J Invest Dermatol 2004;122:337–341.
Omholt K, Platz A, Kanter L, et al. NRAS and BRAF mutations arise early during melanoma pathogenesis and are preserved throughout tumor progression. Clin Cancer Res 2003;9:6483–6488.
Gorden A, Osman I, Gai W, et al. Analysis of BRAF and N-RAS mutations in metastatic melanoma tissues. Cancer Res 2003;63:3955–3957.
Maldonado JL, Fridlyand J, Patel H, et al. Determinants of BRAF mutations in primary melanomas. J Natl Cancer Inst 2003;95:1878–1880.
van Dijk MC, Bernsen MR, Ruiter DJ . Analysis of mutations in B-RAF, N-RAS, and H-RAS genes in the differential diagnosis of Spitz nevus and Spitzoid melanoma. Am J Surg Pathol 2005;29:1145–1151.
Gill M, Cohen J, Renwick N, et al. Genetic similarities between Spitz nevus and Spitzoid melanoma in children. Cancer 2004;101:2636–2640.
Saldanha G, Purnell D, Fletcher A, et al. High BRAF mutation frequency does not characterize all melanocytic tumor types. Int J Cancer 2004;111:705–710.
Mihic-Probst D, Perren A, Schmid S, et al. Absence of BRAF gene mutations differentiates Spitz nevi from malignant melanoma. Anticancer Res 2004;24:2415–2418.
Palmedo G, Hantschke M, Rütten A, et al. The T1796A mutation of the BRAF gene is absent in Spitz nevi. J Cutan Pathol 2004;31:266–270.
Gill M, Renwick N, Silvers DN, et al. Lack of BRAF mutations in Spitz nevi. J Invest Dermatol 2004;122:1325–1326.
Turner DJ, Zirvi MA, Barany F, et al. Detection of the BRAF V600E mutation in melanocytic lesions using the ligase detection reaction. J Cutan Pathol 2005;32:334–339.
Toussaint S, Kamino H . Dysplastic changes in different types of melanocytic nevi. A unifying concept. J Cutan Pathol 1999;26:84–90.
Barnhill RL . Spitz nevi and variants. In: Barnhill RL (ed). Pathology of Melanocytic Nevi and Malignant Melanoma. Butterworth-Heinemann: Newton, MA, 1995, pp 97–130.
Greenson JK, Bonner JD, Ben-Yzhak O, et al. Phenotype of microsatellite unstable colorectal carcinomas: well-differentiated and focally mucinous tumors and the absence of dirty necrosis correlate with microsatellite instability. Am J Surg Pathol 2003;27:563–570.
Bergman R, Dromi R, Trau H, et al. The pattern of HMB-45 antibody staining in compound Spitz nevi. Am J Dermatopathol 1995;17:542–546.
Palazzo J, Duray PH . Typical, dysplastic, congenital and Spitz nevi: a comparative immunohistochemical study. Hum Pathol 1989;20:341–346.
Bergman R, Malkin L, Sabo E, et al. MIB-1 monoclonal antibody to determine proliferative activity of Ki-67 antigen as an adjunct to the histopathologic differential diagnosis of Spitz nevi. J Am Acad Dermatol 2001;44:500–504.
Li LX, Crotty KA, McCarthy SW, et al. A zonal comparison of MIB1-Ki67 immunoreactivity in benign and malignant melanocytic lesions. Am J Dermatopathol 2000;22:489–495.
Kanter-Lewensohn L, Hedblad MA, Wejde J, et al. Immunohistochemical markers for distinguishing Spitz nevi from malignant melanomas. Mod Pathol 1997;10:917–920.
Niemann TH, Argenyi ZB . Immunohistochemical study of Spitz nevi and malignant melanoma with use of antibody to proliferating cell nuclear antigen. Am J Dermatopathol 1993;15:441–445.
Tu P, Miyauchi S, Miki Y . Proliferative activities in Spitz nevus compared with melanocytic nevus and malignant melanoma using expression of PCNA/cyclin and mitotic rate. Am J Dermatopathol 1993;15:311–314.
Kapur P, Selim MA, Roy LC, et al. Spitz nevi and atypical Spitz nevi/tumors: a histologic and immunohistochemical analysis. Mod Pathol 2005;18:197–204.
Ribe A, NcNutt NS . S100A6 protein expression is different in Spitz nevi and melanomas. Mod Pathol 2003;16:505–511.
Howat AJ, Giri DD, Cotton DWK, et al. Nucleolar organizer regions in Spitz nevi and malignant melanomas. Cancer 1989;63:474–478.
Tosi P, Miracco C, Santopietro R, et al. Possible diagnostic role of telomerase activity evaluation in the differential diagnosis between Spitz naevi and cutaneous malignant melanoma. Br J Dermatol 2000;142:1060–1061.
Fullen DR, Zhu W, Thomas D, et al. hTERT expression in melanocytic lesions; an immunohistochemical study on paraffin-embedded tissue. J Cutan Pathol 2005;32:680–684.
Guttman-Yassky E, Bergman R, Manov L, et al. Human telomerase RNA component expression in Spitz nevi, common melanocytic nevi, and malignant melanomas. J Cutan Pathol 2002;29:341–346.
Bastian BC, Wesselmann U, Pinkel D, et al. Molecular cytogenetic analysis of Spitz nevi shows clear differences to melanoma. J Invest Dermatol 1999;113:1065–1069.
Poynter JN, Elder JT, Fullen DR, et al. BRAF and NRAS mutations in melanoma and melanocytic nevi. Melanoma Res (in press).
Thomas NE . BRAF somatic mutations in malignant melanoma and melanocytic naevi. Melanoma Res 2006;16:97–103.
Akslen LA, Angelini S, Straume O, et al. BRAF and NRAS mutations are frequent in nodular melanoma but are not associated with tumor cell proliferation or patient survival. J Invest Dermatol 2005;125:312–315.
Carr J, Mackie RM . Point mutations in the N-ras oncogene in malignant melanoma and congenital nevi. Br J Dermatol 1994;131:72–77.
van Elsas A, Zerp SF, van der Flier S, et al. Relevance of ultraviolet-induced N-ras oncogene point mutations in development of primary human cutaneous melanoma. Am J Pathol 1996;149:883–893.
Jiveskog S, Ragnarsson-Olding B, Platz A, et al. N-ras mutations are common in melanomas from sun-exposed skin of humans but rare in mucosal membranes or unexposed skin. J Invest Dermatol 1998;111:757–761.
Demunter A, Stas M, Degreef H, et al. Analysis of N- and K-ras mutations in the distinctive tumor progression phases of melanoma. J Invest Dermatol 2001;117:1483–1489.
Reifenberger J, Knobbe CB, Sterzinger AA, et al. Frequent alterations of Ras signaling pathway genes in sporadic malignant melanomas. Int J Cancer 2004;109:377–384.
Bastian BC, LeBoit PE, Pinkel D . Mutations and copy number increase of HRAS in Spitz nevi with distinctive histopathological features. Am J Pathol 2000;157:967–972.
Acknowledgements
This work was supported in part by NCI U01 CA83180 (SBG) and NIH T32 HG00040 (JNP), as well as a generous gift from Lewis and Lillian Becker. JTE and RPN were supported by the Babcock Memorial Trust. JTE is supported by the Ann Arbor Veterans Affairs Hospital.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fullen, D., Poynter, J., Lowe, L. et al. BRAF and NRAS mutations in spitzoid melanocytic lesions. Mod Pathol 19, 1324–1332 (2006). https://doi.org/10.1038/modpathol.3800653
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800653
Keywords
This article is cited by
-
Spitz melanoma is a distinct subset of spitzoid melanoma
Modern Pathology (2020)
-
New and evolving concepts of melanocytic nevi and melanocytomas
Modern Pathology (2020)
-
Reevaluation of established and new criteria in differential diagnosis of Spitz nevus and melanoma
Archives of Dermatological Research (2018)
-
Mutational dichotomy in desmoplastic malignant melanoma corroborated by multigene panel analysis
Modern Pathology (2015)