Abstract
Malignant rhabdoid tumors are high-grade neoplasms of the central nervous system (CNS), kidneys and soft tissue that usually occur in children. The histologic diagnosis of malignant rhabdoid tumor depends on identification of characteristic rhabdoid cells—large cells with eccentrically located nuclei and abundant, eosinophilic cytoplasm—and immunohistochemistry with antibodies to vimentin, keratin and epithelial membrane antigen. In most malignant rhabdoid tumors, the SMARCB1/INI1 gene, located in chromosome band 22q11.2, is inactivated by deletions and/or mutations, so genetic diagnosis is often possible. However, tissue may not be available for genetic analysis or studies not confirmatory. We assessed SMARCB1/INI1 expression in 17 rhabdoid tumors and 57 other tumors of the CNS, kidney or soft tissue using immunohistochemistry. In total, 12 brain, three renal and two soft tissue rhabdoid tumors were examined along with four glioblastomas, four pilocytic astrocytomas, four oligodendrogliomas, two ependymomas, two choroid plexus papillomas, five pituitary adenomas, four germinomas, four renal carcinomas with Xp11.2 translocations, two clear cell sarcomas, two Wilms' tumors, one renal medullary carcinoma, two desmoplastic small round cell tumors, two alveolar rhabdomyosarcomas, two embryonal rhabdomyosarcomas, one low-grade chondrosarcoma, two extraskeletal myxoid chondrosarcomas, one mesenchymal chondrosarcoma, four malignant peripheral nerve sheath tumors, five metastatic carcinomas and four epithelioid sarcomas, two primary and two metastatic. The neoplastic cells of all rhabdoid tumors, the four epithelioid sarcomas and the renal medullary carcinoma did not express SMARCB1/INI1 by immunohistochemistry; neoplastic cells of all other tumors expressed SMARCB1/INI1. Immunohistochemistry to assess expression of SMARCB1/INI1 may be useful in the diagnosis of rhabdoid tumors of the CNS, kidneys and soft tissue.
Similar content being viewed by others
Main
Malignant rhabdoid tumors are highly aggressive neoplasms that usually occur in the central nervous system (CNS), kidneys and soft tissue of children.1 The histologic diagnosis of malignant rhabdoid tumor is based on the observation of medium-sized to relatively large cells with eccentrically located nuclei and abundant cytoplasm, often with paranuclear filamentous inclusions, and a polyphenotypic immunoprofile with frequent expression of vimentin, keratin and epithelial membrane antigen. In most malignant rhabdoid tumors, the SMARCB1/INI1 gene, located in chromosome band 22q11.2, is inactivated by deletions and/or mutations, so genetic diagnosis is often possible.2, 3, 4, 5, 6, 7, 8 Sometimes, though tissue is not available for genetic analysis or studies are not confirmatory.8
We used immunohistochemistry to assess SMARCB1/INI1 expression in malignant rhabdoid tumors of the kidney and soft tissue and atypical teratoid/rhabdoid tumors, the analagous CNS tumors. We also examined expression of SMARCB1/INI1 in a variety of pediatric kidney tumors and adult and pediatric brain and soft tissue tumors to assess the role of SMARCB1/INI1 immunohistochemistry in diagnosis.
Materials and methods
Seventy-four tumors—12 brain, three renal and two soft tissue rhabdoid tumors, four glioblastomas, four pilocytic astrocytomas, four oligodendrogliomas, one WHO grade II and three WHO grade III, two ependymomas, two choroid plexus papillomas, five pituitary adenomas, four germinomas, four renal carcinomas with Xp11.2 translocations, two clear cell sarcomas, two Wilms' tumors, one renal medullary carcinoma, two desmoplastic small round cell tumors, two alveolar rhabdomyosarcomas, two embryonal rhabdomyosarcomas, one low-grade chondrosarcoma, two extraskeletal myxoid chondrosarcomas, one mesenchymal chondrosarcoma, four malignant peripheral nerve sheath tumors, five metastatic carcinomas and four epithelioid sarcomas, two primary and two metastatic, one of each in two patients—were selected from the surgical pathology files of the Departments of Pathology of Children's Medical Center and the University of Texas Southwestern Medical Center. The tumors were chosen to provide an indication of the specificity of expression of SMARCB1/INI1 among CNS, kidney and soft tissue neoplasms and individual cases selected if enough tissue was available for confirmation of the diagnosis and additional immunohistochemical stains. In addition, equivocal, focal or unexpected staining of some tumors reported by Judkins et al9 prompted inclusion of several examples of oligodendrogliomas and pituitary adenomas. The diagnoses were based on assessment of clinical data and examination of hematoxylin and eosin-stained sections, usually other immunohistochemically stained sections and, in some cases, other ancillary studies. Specifically, for CNS glial tumors, immunohistochemistry was used in selected cases to identify expression of glial fibrillary acidic protein, microtubule-associated protein 2, prealbumin and Ki-67. For germinomas, immunohistochemistry was used to identify placental alkaline phosphatase and, for pituitary adenomas, chromogranin and/or synaptophysin and some or all anterior pituitary hormones. For case 28, an anaplastic oligodendroglioma, genetic analysis revealed 1p/19q loss of heterozygosity. For the renal carcinomas, cytogenetic analysis identified t(X;1) or t(X;17) translocations. For soft tissue tumors and carcinomas, immunohistochemical stains included those to identify muscle antigens, S100 and keratin.
All staining was performed using a BioTek Solutions TechMate™ 500 system (Ventana Medical Systems, Tucson, AZ, USA) using the ultra-streptavidin biotin system with horseradish peroxidase and diaminobenzidine (DAB) as chromogen (Signet Laboratories, Dedham, MA, USA). Reveal™ (BioCare Medical, Walnut Creek, CA, USA) was used for heat-induced epitope retrieval. Optimum primary antibody dilutions were predetermined using known positive control tissues. A microarray that included sections of normal cerebellum, small cell carcinoma of the lung and colonic adenocarcinoma was included in each staining run. Moreover, in tumor sections, non-neoplastic cells—eg, lymphocytes, fibroblasts, endothelial cells, fat cells, cells of renal tubules and glomeruli, pneumocytes and neurons and glial cells—served as internal positive controls. Not all non-neoplastic cells of a given tissue stained.
Paraffin sections were cut at 3 μm on a rotary microtome, mounted on positively charged glass slides (POP 100 capillary gap slides, Ventana Medical Systems, Tucson, AZ, USA) and air-dried overnight. The sections were deparaffinized in xylene and ethanol, quenched with fresh 3% hydrogen peroxide for 10 min and rinsed with deionized water. For heat-induced epitope retrieval, sections were placed in 200 ml Reveal™ citrate buffer, boiled in a circulating waterbath for 15 min at 100°C then cooled in buffer for 20 min and rinsed in deionized water then buffer. Then, the sections were incubated in unlabeled blocking serum for 15 min (to block nonspecific binding of the secondary antibody), then incubated overnight at 4°C with either BAF47, an antibody to the SMARCB1/INI1 gene product (1:40, BD Biosciences, Chicago, IL, USA) or buffer alone (as a negative reagent control). Following washing in buffer, the sections were incubated for 25 min with a biotinylated polyvalent secondary antibody solution (containing goat antibodies to rabbit and mouse immunoglobulins). Following another buffer wash, the sections were incubated in horseradish peroxidase-conjugated streptavidin–biotin complex for 15 min, washed in buffer, incubated with two changes, 5 min each, of a freshly prepared mixture of DAB and H2O2 in buffer, then washed in buffer, and then in water. Finally, the sections were counterstained with hematoxylin, dehydrated in a graded series of ethanols, then xylene and coverslipped.
Results
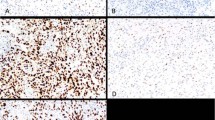
Clinical data and results of SMARCB1/INI1 immunohistochemistry are shown in Tables 1, 2 and 3. Table 1 shows the staining results for 37 primary intracranial tumors. The 12 patients with atypical teratoid/rhabdoid tumors ranged in age from 3 months to 5 years 3 months with a median age of approximately 1 year 5 months. Male to female ratio was 0.83 to 1. Six of 11 tumors occurred in the posterior fossa, three occurred in the cerebral hemispheres and one in the hypothalamus. Expression of SMARCB1/INI1 was not detected by immunohistochemistry in neoplastic cells in the 12 tumors. All or the vast preponderance of neoplastic nuclei were unstained in all cases (Figure 1).
The age range for the other 25 brain tumors was 2–61 years. Nine occurred in the cerebral hemispheres, six in the sella or suprasellar region, two in the posterior fossa, two in the spinal cord and one each in the thalamus, optic nerve, pineal region and 3rd and lateral ventricles. There was expression of SMARCB1/INI1 by neoplastic cells in all tumors. The vast preponderance of neoplastic nuclei were stained in all cases (Figure 2).
Table 2 shows the staining results for 12 childhood kidney tumors. Three patients, two male and one female, ages 8 months, 1 year 6 months and 3 years 7 months had malignant rhabdoid tumors. The age range for the other kidney tumors was 1 year 1 month to 19 years 6 months. There were four renal carcinomas, three with t(X;17) and one with t(X;1), two clear cell sarcomas, two Wilms' tumors and one renal medullary carcinoma. Expression of SMARCB1/INI1 was not detected by immunohistochemistry in neoplastic cells in the rhabdoid tumors or the medullary carcinoma. All or the vast preponderance of neoplastic nuclei were unstained (Figure 3). There was nuclear expression of SMARCB1/INI1 by neoplastic cells in all renal carcinomas and both clear cell sarcomas and Wilms' tumors (Figure 4). In general, the vast preponderance of neoplastic nuclei were stained. In case 45, a renal carcinoma t(X;17), stained nuclei were diffuse, but the percentage was not as great as in the other cases.
Table 3 shows the results for two soft tissue malignant rhabdoid tumors and 23 other soft tissue or metastatic tumors of types in the differential diagnosis of extrarenal rhabdoid tumors. Neoplastic cells of both soft tissue malignant rhabdoid tumors and all four epithelioid sarcomas did not express SMARCB1/INI1 by immunohistochemistry. In all of these cases, all or the vast preponderance of neoplastic nuclei were unstained (Figure 5). There was nuclear expression of SMARCB1/INI1 in neoplastic cells in both desmoplastic small round cell tumors and all rhabdomyosarcomas, chondrosarcomas, carcinomas and malignant peripheral nerve sheath tumors (Figure 5). The vast preponderance of neoplastic nuclei were stained in all cases except the low-grade chondrosarcoma (case 58) and one of the malignant peripheral nerve sheath tumors (case 63). In these cases, like the renal carcinoma (case 45), stained nuclei were diffuse, but the percentage was not as great as in the other cases.
Discussion
The SMARCB1/INI1 gene, that maps to chromosome 22q11.2, is part of the SWI/SNF chromatin remodeling complex that plays a role in transcriptional regulation.10 SMARCB1/INI1 is inactivated homozygously in the majority of malignant rhabdoid tumors by deletions and/or mutations; up to 20% of tumors show no alterations at either the DNA or RNA levels.2, 3, 4, 5, 6, 7, 8 Thus, when informative, genetic analysis is useful for confirmation of the histologic diagnosis of malignant rhabdoid tumor. However, the genetic findings also suggest that immunohistochemistry can be used to identify inactivation of SMARCB1/INI1 and thereby support the morphologic diagnosis of malignant rhabdoid tumor. Absence of expression of SMARCB1/INI1 by immunohistochemistry in tumors of other morphologic types would lessen diagnostic usefulness but raise the possibility of inactivation of the gene in those tumors.
In all, 12 (of 12) tumors that were clinically and morphologically atypical teratoid/rhabdoid tumors showed no nuclear staining by immunohistochemistry with the BAF47 antibody; 25 (of 25) other brain tumors of various types showed nuclear staining. With regard to specificity, we sought (1) to examine expression in tumors that are often considered in the differential diagnosis of cerebral, cerebellar and sellar region tumors in children and adults and (2) to complement Judkins et al9 study. Most CNS tumors in childhood, including atypical teratoid/rhabdoid tumors, occur in the posterior fossa11 and the most common types are neuroglial: astrocytoma (usually pilocytic astrocytoma) primitive neuroectodermal tumor (PNET) and ependymoma. Tumors often considered in the differential diagnosis of atypical teratoid/rhabdoid tumor are PNET, germ cell tumors, choroid plexus carcinoma and ependymoma. The neoplastic cells of all four pilocytic astrocytomas and both ependymomas expressed SMARCB1/INI1 by immunohistochemistry. We also found expression of SMARCB1/INI1 by the neoplastic cells of two choroid plexus papillomas. Judkins et al9 found nuclear expression of SMARCB1/INI1 in 10 (of 10) PNETs. The second most common site for atypical teratoid/rhabdoid tumors in childhood is supratentorial/suprasellar. Pilocytic astrocytomas, craniopharyngiomas and germ cell tumors are among the most common sellar region tumors in children. In addition to the observed expression in pilocytic astrocytomas, the neoplastic cells of all four germinomas expressed SMARCB1/INI1. Judkins et al9 found nuclear staining in two (of two) craniopharyngiomas.
Most CNS tumors in adults occur in or in relation to the cerebral hemispheres and the majority of atypical teratoid/rhabdoid tumors in adults occur in the cerebral hemispheres or sellar region.12 We found nuclear expression of SMARCB1/INI1 in all glioblastomas (four of four) and oligodendrogliomas (four of four), two common types of infiltrating gliomas that occur most frequently in the cerebrum. Judkins et al9 found loss of SMARCB1/INI1 expression by immunohistochemistry in an oligodendroglioma and focal expression in an anaplastic oligoastrocytoma. Their fluorescence in situ hybridization study of the oligodendroglioma showed no chromosome 22 deletion. Although more oligodendrogliomas should be studied, our findings suggest that SMARCB1/INI1 is expressed in these gliomas.
Raisanen et al12 called attention to the relative frequency of atypical teratoid/rhabdoid tumors in the sellar region of adults. The differential diagnosis of sellar region tumors in adults includes pituitary adenoma, five (of five) examples of which showed nuclear expression of SMARCB1/INI1. Judkins et al9 found faint nuclear staining (immunohistochemistry with BAF47) of cells in a single pituitary adenoma. Our findings indicate that SMARCB1/INI1 is expressed in pituitary adenomas.
Altogether, the findings suggest that immunohistochemistry with an antibody to the SMARCB1/INI1 gene product is a sensitive and specific means of identifying atypical teratoid/rhabdoid tumors.
We also studied SMARCB1/INI1 expression in rhabdoid tumors of the kidney with a view toward differential diagnosis. The most common pediatric kidney tumor is Wilms' tumor (nephroblastoma).13 Other kidney tumors that occur in children are mesoblastic nephroma, clear cell sarcoma, rhabdoid tumor, renal medullary carcinoma and renal carcinomas associated with Xp11.2 translocations. All four Wilms' tumors, all four renal carcinomas and both clear cell sarcomas expressed nuclear SMARCB1/INI1 by immunohistochemistry. There was no expression of SMARCB1/INI1 by neoplastic cells of the three malignant rhabdoid tumors. In their study of renal and pediatric soft tissue tumors, Hoot et al6 reported no expression of SMARCB1/INI1 by immunohistochemistry in neoplastic cells of 19 renal malignant rhabdoid tumors. Four (of four) cellular congenital mesoblastic nephromas, four (of four) clear cell sarcomas and six (of six) Wilms' tumors expressed SMARCB1/INI1. With regard to childhood kidney tumors, our findings support those of Hoot et al and suggest that absence of SMARCB1/INI1 expression by immunohistochemistry distinguishes malignant rhabdoid tumors from most common pediatric kidney tumors, including renal carcinomas associated with Xp11.2 translocations.
The tumor cells of a single case of renal medullary carcinoma did not express SMARCB1/INI1 by immunohistochemistry. Renal medullary carcinoma is a comparatively rare kidney tumor that usually occurs in patients 10–40 years old with sickle cell trait.14 The neoplastic cells are poorly differentiated with clear nuclei and prominent nucleoli and sometimes have rhabdoid morphology.14 There are few reports describing the cytogenetics of renal medullary carcinomas.14, 15, 16 Swartz et al14 reported a case with loss of chromosome 22 and Stahlschmidt et al16 a case with a near tetraploid clone with all four 22 chromosomes with an abnormality of 22q11. Although only one case of medullary carcinoma was examined, the finding of loss of expression of SMARCB1/INI1 by immunohistochemistry raises the possibility of inactivation of the SMARCB1/INI1 gene in at least some of these tumors. More studies of SMARCB1/INI1 in renal medullary carcinomas are warranted.
The rhabdoid cells in two soft tissue malignant rhabdoid tumors did not express SMARCB1/INI1 by immunohistochemistry. A total of 19 other tumors of types in the differential diagnosis of extrarenal rhabdoid tumor expressed the protein—both desmoplastic small round cell tumors and all rhabdomyosarcomas, chondrosarcomas, carcinomas and malignant peripheral nerve sheath tumors. Hoot et al6 reported no expression of SMARCB1/INI1 by immunohistochemistry in neoplastic cells of eight (of eight) extrarenal malignant rhabdoid tumors; neoplastic cells of 13 (of 13) Ewing's sarcomas, five (of five) desmoplastic small round cell tumors, five (of five) rhabdomyosarcomas and two (of two) epithelioid sarcomas expressed SMARCB1/INI1. Perry et al17 found nuclear expression of SMARCB1/INI1 by immunohistochemistry in neoplastic cells of three (of three) melanomas.
Thus, as in the CNS and kidneys, absence of expression of SMARCB1/INI1 by immunohistochemistry helps to identify soft tissue malignant rhabdoid tumors.
In Hoot et al study6 of two epithelioid sarcomas, nuclear staining was diffuse and strong in one, but focal and variable in the other. We found absence of expression of SMARCB1/INI1 by immunohistochemistry in both primary and metastatic epithelioid sarcomas in two patients. The absence of expression of SMARCB1/INI1 is interesting in light of recent cytogenetic and molecular genetic studies by Modena et al18 that identified deletions of SMARCB1/INI1 in 5 of 11 so-called ‘proximal type’ epithelioid sarcomas. All five cases and one additional conventional or ‘distal type’ (six of 11) demonstrated inactivation at the protein level.18 So, in regard to differential diagnosis, absence of expression of the SMARCB1/INI1 protein may not help to distinguish malignant rhabdoid tumors from epithelioid sarcomas.
Since malignant rhabdoid tumors and epithelioid sarcomas are related by similar morphology and immunophenotype, the finding of inactivation of the SMARCB1/INI1 tumor suppressor gene in some cases of each type raises the possibility that they are entities in a spectrum.
The diagnoses of atypical teratoid/rhabdoid tumor and malignant rhabdoid tumor may be difficult because of the rarity of the tumors, diverse histology and polyphenotypic immunoprofiles with antigen expression that is usually multifocal. Immunohistochemical identification of absence of expression of SMARCB1/INI1 may help to distinguish atypical teratoid/rhabdoid tumors from other intracranial tumors and malignant rhabdoid tumors from other common pediatric kidney tumors and adult and pediatric soft tissue tumors.
References
Schofield D . Extrarenal rhabdoid tumour. In: Fletcher CDM, Unni KK, Mertens F (eds). Pathology & Genetics: Tumors of Soft Tissue and Bone. IARC Press: Lyon, 2004, pp 219–220.
Biegel JA, Rorke LB, Packer RJ, et al. Monosomy 22 in rhabdoid or atypical tumors of the brain. J Neurosurg 1990;73:710–714.
Biegel JA, Zhou JY, Rorke LB, et al. Germ-line and acquired mutations of INI1 in atypical teratoid and rhabdoid tumors. Cancer Res 1999;59:74–79.
Biegel JA, Kalpana G, Knudsen ES, et al. The role of INI1 and SWI/SNF complex in the development of rhabdoid tumors: meeting summary from the Workshop on Childhood Atypical Teratoid/Rhabdoid Tumors. Cancer Res 2002;62:323–328.
Biegel JA, Tan L, Zhang F, et al. Alterations of the hSNF5/INI1 gene in central nervous system atypical teratoid/rhabdoid tumors and renal and extrarenal rhabdoid tumors. Clin Cancer Res 2002;8:3461–3467.
Hoot AC, Russo P, Judkins AR, et al. Immunohistochemical analysis of hSNF5/INI1 distinguishes renal and extra-renal malignant rhabdoid tumors from other pediatric soft tissue tumors. Am J Surg Pathol 2004;28:1485–1491.
Rousseau-Merck MF, Versteege I, Legrand I, et al. hSNF5/INI1 inactivation is mainly associated with homozygous deletions and mitotic recombinations in rhabdoid tumors. Cancer Res 1999;59:3152–3156.
Versteege I, Sevenet N, Lange J, et al. Truncating mutations of hSNF5/INI1 in aggressive paediatric cancer. Nature 1998;394:203–206.
Judkins AR, Mauger J, Rorke LB, et al. Immunohistochemical analysis of hSNF5/INI1 in pediatric CNS neoplasms. Am J Surg Pathol 2004;28:644–650.
Weber M, Stockhammer F, Schmitz U, et al. Mutational analysis of INI1 in sporadic human brain tumors. Acta Neuropathol 2001;101:479–482.
Ironside JW, Moss TH, Louis DN, et al. Diagnostic Pathology of Nervous System Tumors. Churchill Livingstone: London, 2002.
Raisanen J, Biegel JA, Hatanpaa KJ, et al. Chromosome 22q deletions in atypical teratoid/rhabdoid tumors in adults. Brain Pathol 2005;15:23–28.
Perlman EJ, Grosfeld JL, Togashi K, et al. Nephroblastoma. In: Eble JN, Sauter G, Epstein JI, et al (eds). Pathology & Genetics: Tumors of the Urinary System and Male Genital Organs. IARC Press: Lyon, 2004, pp 48–52.
Swartz MA, Karth J, Schneider DT, et al. Renal medullary carcinoma: clinical, pathologic, immunohistochemical and genetic analysis with pathogenetic implications. Urology 2002;60:1083–1089.
Avery RA, Harris JE, Davis Jr CJ, et al. Renal medullary carcinoma: clinical and therapeutic aspects of a newly described tumor. Cancer 1996;78:128–132.
Stahlschmidt J, Cullinane C, Roberts P, et al. Renal medullary carcinoma: prolonged remission with chemotherapy, immunohistochemical characterisation and evidence of bcr/abl rearrangement. Med Pediatr Oncol 1999;33:551–557.
Perry A, Fuller CE, Judkins AR, et al. INI1 expression is retained in composite rhabdoid tumors, including rhabdoid meningiomas. Mod Pathol 2005;18:951–958.
Modena P, Lualdi E, Facchinetti F, et al. SMARCB1/INI1 tumor suppressor gene is frequently inactivated in epithelioid sarcomas. Cancer Res 2005;65:4012–4019.
Acknowledgements
This study was supported by a grant from the Children's Brain Tumor Foundation of the Southwest (JR) and a grant from the Henry Foundation to the Annette G Strauss Center for Neurooncology (JR).
Author information
Authors and Affiliations
Corresponding author
Additional information
This paper was presented in part at the 94th Annual Meeting of the United States and Canadian Academy of Pathology in San Antonio, TX, February 26–March 4, 2005.
Rights and permissions
About this article
Cite this article
Sigauke, E., Rakheja, D., Maddox, D. et al. Absence of expression of SMARCB1/INI1 in malignant rhabdoid tumors of the central nervous system, kidneys and soft tissue: an immunohistochemical study with implications for diagnosis. Mod Pathol 19, 717–725 (2006). https://doi.org/10.1038/modpathol.3800581
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800581
Keywords
This article is cited by
-
Differential expression profiling of onco and tumor-suppressor genes from major-signaling pathways in Wilms’ tumor
Pediatric Surgery International (2022)
-
Update on selected advances in the immunohistochemical and molecular genetic analysis of soft tissue tumors
Virchows Archiv (2020)
-
Expression of cyclin D1 in clear cell sarcoma of kidney. Is it useful in differentiating it from its histological mimics?
Diagnostic Pathology (2019)
-
Seltene kindliche Nierentumoren
Der Pathologe (2019)
-
A mosaic pattern of INI1/SMARCB1 protein expression distinguishes Schwannomatosis and NF2-associated peripheral schwannomas from solitary peripheral schwannomas and NF2-associated vestibular schwannomas
Child's Nervous System (2017)