Abstract
Dysgerminoma is the most common malignant ovarian germ cell tumor and shares histological and immunophenotypical features with its testicular counterpart, seminoma. Chromosome 12p abnormalities are genetic hallmarks of testicular seminomas. Little is known about these genetic changes in dysgerminoma. We performed dual color fluorescence in situ hybridization (FISH) analyses with a centromeric α-satellite probe for chromosome 12 and a subtelomeric probe for 12p on paraffin-embedded tissue sections from 21 dysgerminomas and two gonadoblastomas. Chromosome 12p abnormalities were detected in 81% of dysgerminomas. In all, 57% of cases had only isochromosome 12p and 5% had only 12p overrepresentation. In all, 19% had both isochrome 12p and 12p overrepresentation. Gonadoblastomas were negative for isochromosome 12p or 12p overrepresentation. Chromosome 12p abnormalities are common in dysgerminoma of the ovary. FISH analyses for chromosome 12p abnormalities may be a useful diagnostic adjunct for confirming the diagnosis of dysgerminoma and for distinguishing it from nongerm cell malignancies that enter into the differential diagnosis.
Similar content being viewed by others
Main
Ovarian germ cell tumors comprise a heterogeneous group of benign and malignant neoplasms, among which dysgerminoma is the most common malignant tumor. Dysgerminoma represents the ovarian counterpart of testicular seminoma.1 Unlike seminoma, dysgerminoma has not been well characterized genetically due to the scarcity of cases and the difficulty in obtaining fresh material necessary to perform classical cytogenetic analyses.
Gain of genetic material from the short arm of chromosome 12 is an early molecular event in the evolution of testicular germ cell tumors, and there is additional evidence that 12p overrepresentation is involved in the development of invasive potential.2, 3, 4, 5 The hallmark genetic marker of testicular germ cell tumor is the presence of an isochromosome of the short arm of chromosome 12 (i(12p)), first recognized using karyotypic analysis by Atkin and Baker6, 7 who described a small metacentric marker in a small series of seminomas and subsequently in nonseminomatous germ cell tumors. Very little is known about the genetic abnormalities of dysgerminoma. In this study, we analyze chromosome 12p abnormalities in a series of 21 ovarian dysgerminomas and two gonadoblastomas with dual-color fluorescence in situ hybridization (FISH) techniques on formalin-fixed, paraffin-embedded specimens.
Materials and methods
Specimens
A total of 21 cases of ovarian dysgerminomas were retrieved from the files of the Departments of Pathology of three different Institutions (Indiana University Medical Center, Indianapolis, IN, USA; University Hospitals of Cleveland, Clevleland, OH, USA; and Yale University, New Haven, CT, USA). The patients’ ages ranged from 10 and 50 years (mean, 23 years). The FIGO staging system for ovarian neoplasms was used.8 Pathological stage was recorded as 1a in 14 cases, 1c in one case, 2a in one case and 3c in the remaining five cases, among which four had lymph node metastases and one showed peritoneal disease. Histologically, all of the tumors were pure dysgerminomas. Additionally, two cases of gonadoblastoma from a 13-year-old and a 15-year-old patients were evaluated in this study.
Tissue Preparation and Fluorescence In Situ Hybridization
Fluorescence in situ hybridization (FISH) was performed as previously described.9, 10, 11, 12 Sections (4 μm) were prepared from buffered, formalin-fixed, paraffin-embedded tissue blocks. The slides were deparaffinized with two washes of xylene, 15 mins for each, and the slides were subsequently washed twice with absolute ethanol, 10 min each. The slides then were air dried in the hood. Next the slides were treated in 0.1 mM citric acid (pH6.0) (Zymed, CA, USA) at 95°C for 10 min, rinsed in distilled water for 3 min, and followed by a wash of 2 × SSC (standard saline citrate) for 5 min. Digestion of the tissue was performed by applying 0.4 ml of pepsin (5 mg/ml in 0.9% NaCl, pH 1.5) (Sigma, St Louis, MO, USA) at 37°C for 40 min. The slides were rinsed with distilled water for 3 min and further washed with 2 × SSC for 5 min, and then allowed to air dry.
Dual-color FISH was performed by using a mixture of a Spectrum orange-labeled Centromeric α-satellite DNA probe (CEP12) and a Spectrum green-labeled subtelomeric (Tel12) DNA probe for chromosome 12p. Both of the probes were from Vysis (Vysis, Downers Grove, IL, USA) and were diluted with tDenHyb2 (Insitus, Alburquerque, NM, USA) in a ratio of 1:50 and 1:20, respectively. Diluted probes (5 μl) were added to the slide in the reduced light condition. The slides were covered with a 22 × 22 mm cover slip and sealed with rubber cement. Denaturation was achieved by incubating the slides at 75°C for 10 min in a humidified box and then hybridized at 37°C over night.
The cover slips were removed and the slides were washed extensively twice with 45°C prewarmed 0.1 × SSC/1.5 M urea, 20 min for each. This was followed by a wash with 2 × SSC for 20 min and with 2 × SSC/0.1% NP40 for 10 min at 45°C. The slides were then further washed with room temperature 2 × SSC for 5 min. The slides were air dried and counterstained with 10 μl DAPI (Insitus, Albuquerque, NM, USA). The slides were covered and sealed with nail polish.
The slides were examined using a Zeiss Axioplan 2 microscope (Ziess, Göttingen, Germany) with the following filters: SP-100 DAPI, FITC MF-101 for spectrum green (12p) and Gold 31003 for Spectrum orange (CEP12) from Chroma (Chroma, Brattleboro, VT, USA).
The images were acquired with a CCD camera and analyzed with MetaSystem Isis software (MetaSystem, Belmont, MA, USA). Five sequential focus stacks with 0.4 μm interval were acquired and then integrated into a single image in order to reduce thickness related artifacts.
From each tumor section, 100 nuclei were scored for signal from CEP12 (red) and 12p (green) under the fluorescence microscope with × 1000 magnification and the ratio between green and red signals was subsequently calculated. The quantitative criteria to determine 12p overrepresentation were previously described.12
We analyzed the spatial distribution of the green and red signals to detect the specific patterns of signal aggregation consistent with i(12p), as previously reported.13, 14, 15, 16 A classical seminoma specimen was used as a positive control for FISH analyses, and lymphocytes in dysgerminoma specimens were used for the negative control. The positive control specimen represented by a classical seminoma showed both overrepresentation of 12p and the presence of i(12p) in a small percentage of nuclei, while lymphocytes from the background of dysgerminomas were consistently negative for 12p overrepresentation and for i(12p).
Statistical Analysis
Data were analyzed with SAS software (SAS Institute Inc., Cary, NC, USA). The presence of 12p abnormalities were correlated with other clinicopathologic variables. Since some variable is discrete and not continuous, a multivariate logistic regression model was constructed using a backward stepwise selection procedure. The variables were first analyzed by χ2 method and the odd ratio was calculated. The variables were then included into a complete model, and were progressively eliminated in order to obtain the parsimonious model with the best overall predictive power. A P-value <0.05 was considered statistically significant.
Results
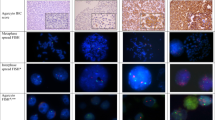
All of the slides showed well-defined hybridization signals. Chromosome 12p abnormalities were detected in 17 of 21 (81%) of dysgerminomas (Figure 1). We observed overrepresentation of 12p in 5 of 21 dysgerminomas (24%), while in 16 of 21 cases (76%) demonstrated i(12p) in a variable percentage of nuclei ranging from 2 to 5%. Among the i(12p)-positive cases, Four cases (25%) also had 12p overrepresentation. Four of five cases (80%) with 12p overrepresentation also had i(12p). Only four of 21 (19%) cases showed neither 12p overrepresentation nor the presence of i(12p). Morphologically, we could not identify difference between dysgerminomas with chromosome 12p abnormalities and dysgerminomas without i(12p) or 12p overrepresentation. FISH results and pathological stages are summarized in Table 1. No correlation was found between 12p abnormalities and various clinicopathologic parameters including pathologic stage (all P-value >0.05).
(a) and (c) Classic dysgerminoma showing typical dysgerminoma cells with well defined cell borders, arranged in nests delimited by fibrovascular septa containing a variable number of small lymphocytes. (b) Fluorescence in situ hybridization displayed two signals (orange) for the chromosome 12 centromeric probe and three signals (green) for 12p, two of which were in close proximity to one centromeric signal, with an aggregation pattern (arrow) consistent with an isochromosome 12p. (d) Fluorescence in situ hybridization showed two signals (orange) for the chromosome 12 centromeric probe and numerous signals for 12p (green) subtelomeric probe, as observed in 12p overrepresentation.
Neither 12p overrepresentation nor the presence of i(12p) was observed in two gonadoblastomas.
Discussion
The hallmark genetic markers of testicular germ cell tumors are chromosome 12p anomalies, including isochromosome 12p and 12p overrepresentation. However, similar genetic markers for ovarian germ cell tumors are less well-characterized. We analyzed a large series of ovarian dysgerminomas by dual color FISH technologies and found 12p abnormalities in 81% of cases. Additionally, we also analyzed two gonadoblatoma for 12p abnormalities using FISH and both were negative for these genetic abnormalities. Our findings suggest that dysgerminoma shows the same genetic markers as its testicular counterpart, the seminoma, and probably shares the same pathogenetic pathways.
There are relatively few reports of genetic abnormalities in dysgerminoma. Jenkyn and McCartney17 reported the results of karyotypic analyses on three different types of malignant ovarian tumors, and i(12p) was detected on ascitic fluid cells from a 19-year-old girl with dysgerminoma. At the same time, Atkin and Baker demonstrated the presence of i(12p) as a peculiar genetic abnormality in one dysgerminoma.18 Dal Cin et al reported a dysgerminoma with overrepresentation of 12p as a small metacentric derivative of chromosome 12.19 Abnormalities of 12p in dysgerminoma were further described by Rodriguez et al20 in a series of three ovarian germ cell tumors and by Riopel et al,21 who reported a gain of 12p in two dysgerminomas. More recently, Kraggerud et al22 confirmed that a gain of 12p was the most common change in dysgerminoma using the comparative genomic hybridization (CGH) approach.
Previous studies have demonstrated that FISH analysis might be helpful in defining 12p overrepresentation as a diagnostic tool in the differential diagnosis of metastatic germ cell tumors.12 An accurate diagnosis of dysgerminoma is important for clinical management as these tumors have a better prognosis than other primary ovarian malignancies, are radiosensitive, and have a good response to cisplatin-based chemotherapy regimens. The differential diagnosis of dysgerminoma includes other histologic types of ovarian germ cell tumors, mainly solid variants of yolk sac tumor and embryonal carcinoma, but also non-germ cell tumors, such as granulosa cell tumor, undifferentiated carcinoma, clear cell adenocarcinoma, and lymphoma, as well as metastatic tumors such as melanoma and breast carcinoma.23 Molecular genetic studies on granulosa cell tumors have frequently identified chromosomal abnormalities as monosomy for chromosome 22 and trisomy for chromosomes 14 and 12,24, 25 but neither i(12p) nor 12p overrepresentation has been reported, whereas extensive genetic characterizations of undifferentiated carcinoma and clear cell adenocarcinomas are still lacking. Positive immunostainings for OCT4 is useful for supporting the diagnosis of dysgerminoma;26 however, a significant proportion of clear cell adenocarcinomas of the ovary are also positive for OCT4.23 We demonstrated that 12p anomalies are detected in the majority of dysgerminomas. Therefore, FISH analysis for 12p anomalies may be a useful diagnostic adjunct in cases where dysgerminoma is considered in the differential diagnosis.
References
Ulbright TM . Germ cell tumors of the gonads: a selective review emphasizing problems in differential diagnosis, newly appreciated, and controversial issues. Mod Pathol 2005;18:S61–S79.
Rosenberg C, Van Gurp RJ, Geelen E, et al. Overrepresentation of the short arm of chromosome 12 is related to invasive growth of human testicular seminomas and nonseminomas. Oncogene 2000;19:5858–5862.
Looijenga LH, Zafarana G, Grygalewicz B, et al. Role of gain of 12p in germ cell tumour development. APMIS 2003;111:161–173.
Ottesen AM, Skakkebaek NE, Lundsteen C, et al. High-resolution comparative genomic hybridization detects extra chromosome arm 12p material in most cases of carcinoma in situ adjacent to overt germ cell tumors, but not before the invasive tumor development. Genes Chromosomes Cancer 2003;38:117–125.
Oosterhuis JW, Looijenga LH . Testicular germ-cell tumours in a broader perspective. Nat Rev Cancer 2005;5:210–222.
Atkin NB, Baker MC . Specific chromosome change, i(12p), in testicular tumours? Lancet 1982;2:1349.
Atkin NB, Baker MC . i(12p): specific chromosomal marker in seminoma and malignant teratoma of the testis? Cancer Genet Cytogenet 1983;10:199–204.
Greene FL, Page DL, Fleming ID, et al. American Joint Committee on Cancer Staging Manual. Springer: New York, 2002.
Brunelli M, Eble JN, Zhang S, et al. Eosinophilic and classic chromophobe renal cell carcinomas have similar frequent losses of multiple chromosomes from among chromosomes 1, 2, 6, 10, and 17, and this pattern of genetic abnormality is not present in renal oncocytoma. Mod Pathol 2005;18:161–169.
Brunelli M, Eble JN, Zhang S, et al. Metanephric adenoma lacks the gains of Chromosomes 7 and 17 and loss of Y which are typical of papillary renal cell carcinoma and papillary adenoma. Mod Pathol 2003;16:1060–1063.
Brunelli M, Eble JN, Martignoni G, et al. Gain of chromosomes 7, 17, 12, 16, and 20, and loss of Y occur early in the evolution of papillary renal cell neoplasia: a fluorescent in situ hybridization study. Mod Pathol 2003;16:1053–1059.
Kernek KM, Brunelli M, Ulbright TM, et al. Fluorescence in situ hybridization analysis of chromosome 12p in paraffin-embedded tissue is useful for establishing germ cell origin of metastatic tumors. Mod Pathol 2004;17:1309–1313.
Coventry S, Punnett HH, Tomczak EZ, et al. Consistency of isochromosome 7q and trisomy 8 in hepatosplenic gammadelta T-cell lymphoma: detection by fluorescence In situ hybridization of a splenic touch-preparation from a pediatric patient. Pediatr Dev Pathol 1999;2:478–483.
Meng FJ, Zhou Y, Giwercman A, et al. Fluorescence in situ hybridization analysis of chromosome 12 anomalies in semen cells from patients with carcinoma in situ of the testis. J Pathol 1998;186:235–239.
Velagaleti GV, Tapper JK, Rampy BA, et al. A rapid and noninvasive method for detecting tissue-limited mosaicism: detection of i(12)(p10) in buccal smear from a child with Pallister-Killian syndrome. Genet Test 2003;7:219–223.
Woodward PJ, Heidenreich A, Looijenga LHJ, et al. Germ cell tumors. In: Eble JN, Sauter G, Epstein JI, Sesterhenn IA (eds). World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. IARC Press: Lyon, 2004, pp 221–249.
Jenkyn DJ, McCartney AJ . A chromosome study of three ovarian tumors. Cancer Genet Cytogenet 1987;26:327–337.
Atkin NB, Baker MC . Abnormal chromosomes including small metacentrics in 14 ovarian cancers. Cancer Genet Cytogenet 1987;26:355–361.
Dal Cin P, Marynen P, Moerman P, et al. Ovarian germ cell tumor with chromosome 12 anomaly but without i(12p). Cancer Genet Cytogenet 1996;91:61–64.
Rodriguez E, Melamed J, Reuter V, et al. Chromosome 12 abnormalities in malignant ovarian germ cell tumors. Cancer Genet Cytogenet 1995;82:62–66.
Riopel MA, Spellerberg A, Griffin CA, et al. Genetic analysis of ovarian germ cell tumors by comparative genomic hybridization. Cancer Res 1998;58:3105–3110.
Kraggerud SM, Szymanska J, Abeler VM, et al. DNA copy number changes in malignant ovarian germ cell tumors. Cancer Res 2000;60:3025–3030.
Cheng L, Thomas A, Roth LM, et al. OCT4: a novel biomarker for dysgerminoma of the ovary. Am J Surg Pathol 2004;28:1341–1346.
Lin YS, Eng HL, Jan YJ, et al. Molecular cytogenetics of ovarian granulosa cell tumors by comparative genomic hybridization. Gynecol Oncol 2005;97:68–73.
Mayr D, Kaltz-Wittmer C, Arbogast S, et al. Characteristic pattern of genetic aberrations in ovarian granulosa cell tumors. Mod Pathol 2002;15:951–957.
Looijenga LH, Stoop H, de Leeuw HP, et al. POU5F1 (OCT3/4) identifies cells with pluripotent potential in human germ cell tumors. Cancer Res 2003;63:2244–2250.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cossu-Rocca, P., Zhang, S., Roth, L. et al. Chromosome 12p abnormalities in dysgerminoma of the ovary: a FISH analysis. Mod Pathol 19, 611–615 (2006). https://doi.org/10.1038/modpathol.3800576
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800576
Keywords
This article is cited by
-
The decisive role of molecular pathology in presumed somatic metastases of type II testicular germ cell tumors: report of 2 cases
Diagnostic Pathology (2020)
-
Multiregion exome sequencing of ovarian immature teratomas reveals 2N near-diploid genomes, paucity of somatic mutations, and extensive allelic imbalances shared across mature, immature, and disseminated components
Modern Pathology (2020)