Abstract
We describe a common, but hitherto not well described, reactive change of the endocervical surface epithelium, commonly seen in association with endometrial carcinoma, and which we term ‘atypical reactive proliferation’. This lesion, especially when florid, has the potential to be misinterpreted as a manifestation of a stage 2A endometrial cancer (endocervical glandular involvement). We examined the cervical sections in 80 consecutive hysterectomy specimens of endometrial cancer. In 22 cases (27.5%), there was cervical involvement by tumour and these cases were excluded from further analysis. Of the remaining cases, atypical reactive proliferation involved the endocervical surface in 40 of 58 (69%) cases, although the degree of abnormality varied widely between individual cases. Histological features characteristic of atypical reactive proliferation (not all features were present in each case) included nuclear stratification and multilayering with short micropapillary processes, squamoid change, hobnail cells and mild cytological atypia. Other features present in some cases were surface erosion, clearing of the cytoplasm, fibrin deposition, an inflammatory cell infiltrate and fibrosis of the subepithelial tissue. In 20 control cases, comprising hysterectomy specimens for benign conditions, similar changes were not seen. Vimentin immunohistochemistry was undertaken in eight cases in which atypical reactive proliferation was particularly florid. Five cases were completely negative and three exhibited very focal positivity. Atypical reactive proliferation involving the endocervical surface is commonly seen in association with endometrial cancer and has the potential to be misinterpreted as endocervical involvement by tumour. Although this could represent a reactive change associated with the presence of an endometrial cancer, we feel atypical reactive proliferation is most likely a reactive/reparative response to recent endometrial biopsy or curettage. The vimentin-negative immunophenotype may be of value in cases where the uterine carcinoma is endometrioid in type as these neoplasms are generally vimentin positive.
Similar content being viewed by others
Main
Endometrial carcinoma is the most common gynaecological malignancy in Western countries. Most tumours are confined to the uterus (FIGO stage 1) at presentation and in these cases the prognosis is usually good, especially in neoplasms with an endometrioid morphology.1 Cervical involvement upstages the tumour to stage 2. In many cases, cervical involvement is not apparent clinically, radiologically or on gross examination of the specimen and is only identified following microscopic examination. This may take the form of endocervical glandular involvement only (FIGO stage 2A) or cervical stromal invasion with or without glandular involvement (FIGO stage 2B). Most cases of cervical stromal involvement are easily recognized microscopically, although a subtle ‘burrowing’ pattern of cervical stromal infiltration has recently been described which can be misdiagnosed as cervical mesonephric remnants or a coexistent premalignant or malignant endocervical glandular lesion.2
Endocervical surface involvement by endometrial carcinoma may be subtle and difficult to recognize, especially if forming a monolayer, and is often only identified on medium—or high-power examination. Consequently, there is a risk of missing microscopic endocervical surface involvement by endometrial cancer and pathologists now actively look for this phenomenon with the result that more FIGO stage 2A endometrial cancers are being identified (WGM—personal unpublished observations). Recently, we have noticed a common change in the endocervical surface epithelium which we term ‘atypical reactive proliferation’ and which has received scant attention in the literature. In this retrospective study, we examined the cervical sections in a series of endometrial cancers to more fully characterize this lesion, which, we feel, has the potential to be over-diagnosed as endocervical involvement by tumour, particularly in those cases where the changes are florid.
Materials and methods
Eighty consecutive cases of endometrial carcinoma were selected from the histopathology archive of Belfast Link Laboratories. All cervical sections and representative endometrial tumour sections from each case were reviewed without knowledge of the original histopathology report. Data recorded included patient's age, histological tumour type and FIGO stage, number of cervical blocks taken, presence or absence of cervical involvement by tumour and presence or absence of the lesion we term atypical reactive proliferation involving the endocervical surface epithelium (this was only looked for in those cases in which the cervix was not involved by tumour). The histological features of atypical reactive proliferation are described below. Cases in which the cervix was involved by tumour were excluded from further analysis. In eight cases, where atypical reactive proliferation was particularly florid, immunohistochemical staining with vimentin was undertaken.
Twenty consecutive hysterectomy specimens were also selected where surgery was performed for benign conditions. These included uterine fibroids (n=10), uterine prolapse (n=5) and menorrhagia (n=5). In these cases, the number of cervical blocks taken was recorded and the endocervix was examined for the features described above. In none of these cases had endometrial sampling been undertaken in the last 6 months.
Results
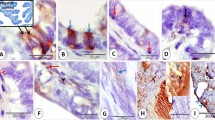
The age of the patients ranged from 21 to 83 years. The FIGO stage was 1A (seven cases), 1B (29 cases), 1C (18 cases), 2A (four cases), 2B (15 cases), 3A (three cases), 3C (three cases) and 4B (one case). The number of cervical sections examined in the cases of endometrial cancer was one (four cases), two (62 cases), three (four cases), four (nine cases) and five (one case). Of the 80 cases of endometrial cancer (69 endometrioid, five serous, four clear cell, two carcinosarcoma), in 22 (27.5%) there was tumour involvement of the cervix and these were excluded from further analysis. Of the remaining 58 cases, in 18 no abnormality of the endocervical surface epithelium was identified. The remaining 40 cases (69%) showed atypical reactive proliferation, although this varied widely in extent and severity. In most cases, atypical reactive proliferation involved the endocervix proximal to the transformation zone while in some cases the lesion was also present at the transformation zone. Consistent features present in most cases were nuclear stratification and multilayering with tufting and short micropapillary processes (Figures 1 and 2). There was associated mild nuclear atypia and in some cases occasional mitotic figures were identified. In some cases, focally the endocervical cells had a squamoid or hobnail appearance (Figure 3) and occasionally there was cytoplasmic clearing. Other features present in a minority of cases were a scattered chronic inflammatory cell infiltrate, surface erosion, the presence of fibrinoid material (Figure 4), stromal vascularization (Figure 5) and stromal hyalinization. In a few cases, small groups of cells similar to those seen on the surface were incorporated into the fibrous stroma and came to lie subepithelially (Figure 6).
In five of the eight cases, where vimentin staining was performed, atypical reactive proliferation was completely negative (Figure 7). Three cases exhibited focal vimentin positivity, but in all these cases the lesion was predominantly negative.
Examination of the cervical sections from the 20 benign hysterectomy specimens (all cases contained two cervical sections) did not reveal features of atypical reactive proliferation.
Discussion
Judging by the cases we have examined, the lesion we term atypical reactive proliferation of the endocervical surface epithelium appears to be extremely common in association with cancer of the uterine corpus, although the extent and severity of the lesion varies widely from case to case. The significance of this lesion lies in its potential to be mistaken for endocervical surface involvement by endometrial carcinoma. In many institutions, FIGO stage 2A endometrial cancers (invasion of the cervix limited to glandular involvement only) are being diagnosed with increasing frequency (WGM—personal unpublished observations), largely owing to the fact that there is an increasing recognition by pathologists of subtle endocervical epithelial involvement by endometrial cancer. Pathologists are nowadays more likely to examine multiple sections from the cervix and to scrutinize the endocervical epithelium carefully under high power. Under these circumstances, there is a risk of overinterpretation of the lesion we describe as tumour involvement. In this retrospective study, we had no knowledge of the histopathology report and thus we do not know if any of the cases were misinterpreted as cervical involvement by tumour, although we suspect some were. Although many histopathologists are likely to be familiar with the lesion we describe herein, we feel the features are worth reporting since they have not been well documented in the literature.
There has been only brief mention in the literature of similar changes to those we describe involving the endocervix in association with endometrial cancer.3 Jordan and Al-Nafussi described a lesion they termed ‘early immature metaplasia with micropapillary surface configuration’ involving the endocervical glands and speculated that this may be a reaction to the presence of an endometrial cancer.3 Their cases were reported in a clinical journal, but judging by the photomicrographs and the pathological description the features they describe are basically similar to those we report herein. They identified these features in 43% of hysterectomies performed for endometrial cancer and also drew attention to subtle endocervical glandular involvement by tumour and the difficulties in distinguishing luminal tumour ‘migrants’ implanted on or attached to the endocervical epithelium from tumour involvement.3, 4
When atypical reactive proliferation is florid there is a risk that it may be mistaken for endocervical epithelial involvement by endometrial cancer. This would result in upstaging of the tumour and possibly the administration of unnecessary radiotherapy. With tumour involvement, the constituent cells are generally more hyperchromatic and atypical and often form glandular structures. Mitotic figures are likely to be more numerous with tumour involving the endocervical epithelium, although in some cases mitoses are not identified and conversely mitotic figures were present in some of our cases of atypical reactive proliferation. We performed vimentin immunohistochemistry in eight cases, where the changes of atypical reactive proliferation were particularly florid. In five cases, the cytoplasm of the epithelial cells was completely negative while three examples exhibited focal positivity, although most of the lesion was negative. Endometrioid adenocarcinomas of the uterine corpus usually exhibit cytoplasmic vimentin positivity.5, 6 Therefore, in those cases where the associated uterine tumour is endometrioid in type, vimentin staining may be of value in excluding tumour involvement of the cervix. Most other histological types of uterine carcinoma, including serous and clear cell, are vimentin negative.
There are at least two possible explanations for the atypical endocervical epithelial changes we describe. The first is that this is a reactive change to the presence of nearby tumour as a result of a paracrine phenomenon. This hypothesis was suggested in the study of Jordan and Al-Nafussi discussed previously.3 However, we consider this theory unlikely and feel that a more plausible explanation is that atypical reactive proliferation is secondary to a recent endometrial biopsy or curettage. It is probable that atypical reactive proliferation is more common and the changes more florid following endometrial curettage rather than outpatient biopsy. However, we have no knowledge in each individual case whether the preoperative diagnostic specimen comprised an outpatient biopsy or curettage and thus cannot confirm this.
In summary, we describe a common phenomenon involving the endocervical surface epithelium in association with endometrial cancer which we term atypical reactive proliferation. We feel this lesion is most likely a reaction to recent endometrial sampling. Pathologists should be aware of the morphological features of this lesion since, particularly when florid, there is a potential for atypical reactive proliferation to be misdiagnosed as endocervical surface involvement by endometrial carcinoma resulting in upstaging of the tumour and possibly the administration of unnecessary adjuvant therapy. In those cases where the uterine tumour is endometrioid in type, immunohistochemical staining with vimentin may be of value.
References
Abeler VM, Kjordstad KE, Berle E . Carcinoma of the endometrium in Norway: a histopathological and prognostic survey of a total population. Int J Gynecol Cancer 1992;2:9–22.
Tambouret R, Clement PB, Young RH . Endometrial endometrioid adenocarcinoma with a deceptive pattern of spread to the uterine cervix: a manifestation of stage IIb endometrial carcinoma liable to be misinterpreted as an independent carcinoma or a benign lesion. Am J Surg Pathol 2003;27:1080–1088.
Jordan LB, Al Nafussi A . Clinicopathological study of the pattern and significance of cervical involvement in cases of endometrial adenocarcinoma. Int J Gynecol Cancer 2002;12:42–48.
Fanning J, Alvarez PM, Tsukada Y, et al. Cervical implantation metastasis by endometrial adenocarcinoma. Cancer 1991;68:1335–1339.
McCluggage WG, Sumathi VP, McBride HA, et al. A panel of immunohistochemical stains, including carcinoembryonic antigen, vimentin, and estrogen receptor, aids the distinction between primary endometrial and endocervical adenocarcinomas. Int J Gynecol Pathol 2002;21:11–15.
Castrillon DH, Lee KR, Nucci MR . Distinction between endometrial and endocervical adenocarcinoma: an immunohistochemical study. Int J Gynecol Pathol 2002;21:4–10.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Scott, M., Lyness, R. & McCluggage, W. Atypical reactive proliferation of endocervix: a common lesion associated with endometrial carcinoma and likely related to prior endometrial sampling. Mod Pathol 19, 470–474 (2006). https://doi.org/10.1038/modpathol.3800556
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800556