Abstract
This study was undertaken to determine the prognostic relevance of the proliferation rate in neoplastic cells in children and adolescents with Hodgkin's lymphoma. Paraffin-embedded biopsy specimens were immunostained with the proliferation-associated monoclonal antibodies Ki-S5 (Ki-67 antigen) and Ki-S2 (which detects the repp86 protein). Repp86 is a protein of about 100 kDa encoded by a gene located on human chromosome band 20q11.2. In contrast to the Ki-67 antigen, repp86 expression is restricted to the cell cycle phases G2, S and M. Immunohistochemical results on diagnostic lymph node biopsy specimens from 224 patients included in two pediatric multicenter Hodgkin's trials, GPOH HD-90 and HD-95, were compared with clinical data. High Ki-67 antigen expression was a striking feature of Hodgkin's and Reed–Sternberg cells as well as lymphocytic and histiocytic cells (median: 80%, range: 20–100%), in contrast to low repp86 expression (median: 20%, range: 10–80%; P<0.001). The proliferation rate was independent of histological subtype, stage and presence of B symptoms. The probability of event-free and overall survival (±standard error) of all patients at 5 years was 91.6±2.0 and 98.1±1.0%, respectively. The proliferation rate of tumor cells did not influence the outcome. The difference between Ki-67 and repp86 expression in Hodgkin's and Reed–Sternberg or lymphocytic and histiocytic cells points to a possible cell cycle arrest in the G1 phase, which may explain the obvious paradox of a highly proliferating but slowly growing paucicellular tumor. High Ki-67 expression does not seem to be an adverse prognostic factor in pediatric and adolescent patients with Hodgkin's lymphoma treated by effective risk-adapted chemo-radiotherapy regimens.
Similar content being viewed by others
Main
A high proliferation rate has been shown to be associated with adverse clinical outcome in a variety of malignant hematological disorders, including non-Hodgkin's lymphomas.1, 2, 3, 4, 5 In 1983, Ki-67 was first described as an antigen directed against proliferating cells.6 Clinical classification of non-Hodgkin's lymphomas into high- and low-grade lymphomas has been shown to be mirrored by differences in Ki-67 staining (proliferation rate <30% or >30%).1, 7 Although recent work has better characterized the molecular structure of the Ki-67 antigen, the exact function of this protein is still not fully understood.8, 9 For many years, the applicability of an anti-Ki-67 antibody was confined to snap-frozen tissues. This obstacle has been solved by generation of formalin-resistant antibodies.10, 11
In Hodgkin's lymphoma, high Ki-67 antigen expression has been repeatedly described in Hodgkin's and Reed–Sternberg cells, the putative neoplastic cells of this lymphoma which comprise less than 1% of all cells of the tumor.12, 13, 14, 15 In many cases, however, the clinical behavior of Hodgkin's lymphoma rather resembles a low-grade lymphoma than a high-grade non-Hodgkin's lymphoma.16 This phenomenon could be explained by arrest of Hodgkin's and Reed–Sternberg cells in the G1 phase of the cell cycle, which is of variable duration and comprises up to 50% of the cell cycle length.17 Progression of the cell cycle is controlled by a complex network of cyclin-dependent kinases, cyclins, their regulatory subunits and cyclin-dependent kinase inhibitors. Cyclin-dependent kinases inactivate negative regulators, for example, retinoblastoma protein by phosphorylation, permit exit from G1 and entry to S cell cycle phase. Overexpression of cyclins regulating the transition from G1 to S phase (eg cyclin E) and from G2 to M (eg cyclins A, B1) was reported recently in Hodgkin's lymphoma.18, 19 In 1997, a newly developed monoclonal antibody, designated Ki-S2, was described, which detects a formalin-resistant epitope of a nuclear protein designated repp86.20 This protein has a molecular weight of about 100 kDa and is encoded by a gene located on human chromosome band 20q11.2.21 Repp86 protein is expressed in the cell cycle phases S/G2/M, but not in G1. Thus, it has now become possible to more accurately detect the fraction of proliferating tumor cells.
The aim of this study was (1) to clarify whether a high Ki-67 expression level in Hodgkin's and Reed–Sternberg cells in classical Hodgkin's lymphoma and in lymphocytic and histiocytic cells in nodular lymphocyte predominant Hodgkin's lymphoma corresponds to the actual tumor growth, as determined by repp86 expression, and (2) to analyze the impact of the proliferation rate of neoplastic cells on the clinical outcome in children and adolescents with Hodgkin's lymphoma.
Patients and methods
Patients
Lymph node biopsy specimens from 231 patients (122 boys, 109 girls) with biopsy proven Hodgkin's lymphoma and a median age of 13.7 years (range: 2.2–19.1) were investigated in this study. Complete clinical data including outcome of therapy were available for 224 patients enrolled in two pediatric multicenter Hodgkin's treatment studies, HD-90 (40 boys, 24 girls) and HD-95 (80 boys, 80 girls), carried out by the German Society of Pediatric Oncology and Hematology between January 1991 and August 2001. The clinicopathological characteristics of the patients are depicted in Table 1. The distribution of histological subtypes, stage, age and presence of B symptoms in the patients investigated here are representative of the cohort of patients in the two trials. The details and treatment regimens of these studies have been outlined in detail.22, 23
Briefly, in both treatment protocols, the therapy consisted of combined radio-chemotherapy regimens stratified into three treatment groups (TG) based on clinical stage, presence of B symptoms and extranodal disease. Patients with localized disease (TG 1: stages I, IIA), intermediate stages (TG 2: stages IIEA, IIB, IIIA) and advanced stages (TG 3: stages IIEB, IIIB, IIIE, IV) initially received two, four or six cycles of polychemotherapy, respectively. Girls were treated with two cycles of OPPA (vincristine, prednisone, procarbazine, adriamycine) and boys with two cycles of OEPA (etoposide substituted for procarbazine). This was followed by two cycles (in TG 2) or four cycles (in TG 3) of COPP (cyclophosphamide, vincristine, prednisone, procarbazine). Radiotherapy was directed to involved fields, with dosages ranging from 20 to 35 Gy, depending on the response to chemotherapy. In trial HD-95, radiotherapy was omitted for patients achieving a complete remission after chemotherapy. The median follow-up of the patients studied was 5.8 years (range: 0.3–12.1 years).
Material
Histology and immunohistochemistry
Biopsy specimens of diagnostic tumor tissues sent to the Department of Hematopathology for reference pathology review within the pediatric Hodgkin's multicenter trials were investigated in this study. The diagnosis was established according to the Rye classification and modified according to the WHO criteria, when indicated by means of conventional staining methods (Hemalaun and Eosin, Giemsa).24, 25 The histological subtypes were distributed as follows: nodular lymphocyte-predominant Hodgkin's lymphoma 24 cases (10%), nodular sclerosis Hodgkin's lymphoma 160 (69%), mixed cellularity Hodgkin's lymphoma 44 (19%), lymphocyte-depleted Hodgkin's lymphoma 1 (<1%), lymphocyte-rich classical Hodgkin's lymphoma 1 (<1%) and not classified 1 (<1%). In the nodular sclerosis subgroup, 129 cases (82%) were further classified as Bennett I subtype and 28 (18%) as Bennett II according to the criteria of the British National Lymphoma Investigation.26 In grade II nodular sclerosis, at least 25% of the tumor nodules contain sheets of Hodgkin's and Reed–Sternberg cells, making up more than 50% of the area. In three cases, no further subclassification was performed.
For immunohistochemistry 5 μm thick sections of paraffin-embedded, formalin-fixed tissue were mounted on 3-amino-propyl-triethoxy-silane pretreated slides. After microwave pretreatment27 and peroxidase blocking, the slides were incubated for 60 min at room temperature with the primary antibodies Ki-S5 and Ki-S2, developed in the authors’ laboratory. Ki-S5 is directed against Ki-67 antigen (supernatant, dilution 1:10) and Ki-S2 is directed against repp86 protein (supernatant, dilution 1:20). This was followed by a 30-min incubation with rabbit-anti-mouse antibody (DAKO, Hamburg, Germany, dilution 1:25). Staining was completed with the streptavidin–biotin complex methods and visualized with diaminobenzidine.28 Alternatively, the alkaline phosphatase-anti-alkaline phosphatase technique was used.29 For unequivocal identification of Hodgkin's and Reed–Sternberg cells, and lymphocytic and histiocytic cells, the tumors were investigated with antibodies directed against the CD30 antigen (Ber-H2, DAKO, Hamburg, Germany) and CD20 antigen (L26, DAKO, Hamburg, Germany).30, 31
To evaluate the proliferation rate, the number of Ki-S5 or Ki-S2-positive tumor cells in a minimum of 10 high-power fields was counted. The number of positively immunostained Hodgkin's and Reed–Sternberg cells was compared with the total number of Hodgkin's and Reed–Sternberg cells. In cases of nodular predominant Hodgkin's lymphoma, lymphocytic and histiocytic cells were analyzed in the same manner. The results were rounded to the nearest 10% level. This procedure was chosen for two reasons. First, the tumor cell distribution within the lymphoid tissue was heterogeneous, for example partial infiltration in classical Hodgkin's lymphoma of mixed cellularity type. Second, due to the small number of Hodgkin's and Reed–Sternberg cells, compared with high-grade lymphomas, in most cases no definitively representative tissue section was available. The proliferation rate was expressed in percent, with the median and range given. To more accurately determine the number of proliferating cells, the ratio between Ki-S2 and Ki-S5 expression was calculated as described previously.32
Statistics
For the statistical analysis, the Wilcoxon test and Spearman's correlation were used to compare Ki-S5 and Ki-S2 distribution. For categorical variables, the χ2 test was used. Overall survival was calculated from the time of diagnosis to the time of last contact or death of any cause and event-free survival as the time from diagnosis to first event (progression, relapse, death of any cause) or the time of last contact. The survival analysis was based on the Kaplan–Meier estimator and survival curves were compared by the log-rank test.33, 34 The prognostic relevance of the proliferation markers was also evaluated by Cox regression models. Analyses were performed with SPSS version 11.5.1 software (SPSS Inc., Chicago, IL, USA) or SAS/STAT version 8 software (SAS Statistics Inc., Cary, NC, USA) on a PC.
Results
Study Population
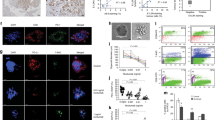
CD30 positivity (Figure 1a) was observed in 192 of 220 (87%) of Hodgkin's lymphoma biopsy specimens.
(a) CD30 expression in a case of mixed cellularity Hodgkin's lymphoma ( × 240, alkaline phosphatase-anti alkaline phosphatase). (b) Proliferation rate of Hodgkin's and Reed–Sternberg cells and reactive lymphocytes in the same lymph node as shown by repp86 expression with some negative tumor cells ( × 240, alkaline phosphatase-anti alkaline phosphatase). (c) Hodgkin's lymphoma with numerous Ki-67-positive Hodgkin's and Reed–Sternberg cells. Atypical mitosis in the center ( × 480, streptavidin–biotin complex). (d) Compared to Ki-67, low repp86 expression indicating the actual proliferation rate. Strong expression in proliferating lymphocytes as internal control ( × 480, alkaline phosphatase-anti alkaline phosphatase). (e) Hodgkin's and Reed–Sternberg cells with strong Ki-67 expression in a case of mixed cellularity Hodgkin's lymphoma ( × 480, streptavidin–biotin complex). (f) Same case as (e) the majority of Hodgkin's and Reed–Sternberg cells are negative for repp86 ( × 480, alkaline phosphatase-anti alkaline phosphatase).
Ki-67 antigen expression was detectable in Hodgkin's and Reed–Sternberg cells and lymphocytic and histiocytic cells in all 231 cases with a median proliferation rate of 80% (range: 20–100%). Ki-S5 resulted in a strong nuclear staining of Hodgkin's and Reed–Sternberg cells and the surrounding reactive T cells (Figure 1c, e). No preference towards a histological subtype was observed (Table 2).
Repp86 expression was also observed in all cases. Ki-S2 staining was confined to the nuclei of proliferating cells. A major finding of this study was that the expression of repp86 protein was significantly lower than that of the Ki-67 antigen, with a median growth fraction of only 20% (Figure 2). The staining intensity was slightly weaker for Ki-S2, but positive and negative Hodgkin's and Reed–Sternberg cells and lymphocytic and histiocytic cells could be differentiated in all cases (Figure 1b, d, f). Similar to the staining results with the Ki-S5 antibody, no differences were detectable between different histological subtypes, clinical stages and presence or absence of B symptoms (Tables 2, 3 and 4).
Classical Hodgkin's Lymphoma
In the nodular sclerosis and mixed cellularity subtypes, more than 95% of the cases were Ber-H2 positive (188 of 193). CD20 was detectable in 21 of 136 (15%) cases of nodular sclerosis and three of 37 (8%) cases of mixed cellularity, respectively.
The median proliferation rate (Ki-67 antigen) in nodular sclerosis was 80% (range: 20–100%) and it was 90% (range: 50–100%) in the mixed cellularity subtype. This difference was not statistically significant. Also, in patients with nodular sclerosis subtypes Bennett I and II, no differences in proliferation rate between the two subtypes were detected. The median repp86 expression in classical Hodgkin's lymphoma was 20% (range: 10–80%).
Nodular Lymphocyte Predominant Hodgkin's Lymphoma
Only 1 of 24 cases with nodular lymphocyte-predominant Hodgkin's lymphoma was CD30 positive in contrast to 190 of 195 cases with classical Hodgkin's lymphoma (P<0.001). CD20 was detected in all 24 cases of nodular lymphocyte-predominant Hodgkin's lymphoma, compared with 24 of 175 cases (14%) with classical Hodgkin's lymphoma (P<0.001). The cytology of the large atypical blasts was classified in all cases as lymphocytic and histiocytic or Popcorn cells.
In this group of 24 patients, the median proliferation rate for Ki-67 antigen was 90% (range: 20–100%), while it was only 20% (range: 10–80%) as determined by immunostaining with the Ki-S2 antibody.
Correlation of Immunohistochemical Findings with Clinical Data and Outcome
The clinical data on 224 patients enrolled into the two most recent treatment protocols, HD-90 and HD-95, were evaluated. The median proliferation rates, as determined by expression of the Ki-67 antigen and repp86 protein, were 80 and 20%, respectively, in both studies. With regard to the growth fraction, no differences were observed between male and female patients. The proliferation rate in this study did not differ between localized (stage I/II) and advanced (stage III/IV) disease (Table 3). Median percentage of Ki-S5-positive Hodgkin's and Reed–Sternberg cells and lymphocytic and histiocytic cells was 80% (range: 20–100%) in patients with B symptoms and 80% (range: 20 to 100%) for those without (Table 4). Patients with extranodal disease (data not shown) did not show higher proliferation rates than those without. The median repp86 protein expression of patients with and without B symptoms was 20%. An analysis of the proliferation rate, as assessed by Ki-67 antigen expression according to the treatment arm to which the patients were allocated, yielded the same results, namely 80% (range: 20–100%) for treatment groups 1, 2 and 3. The repp86 protein expression was 20% (range: 10–80%), 20% (range: 10–70%) and 20% (range: 10–50%), respectively, for the three treatment arms.
At a median follow-up of 5.8 years, 220 of 224 patients (98.2%) were alive. In all, 19 patients (8.5%) had experienced an event, which in two cases was unrelated to Hodgkin's lymphoma. The probabilities of overall and event-free survival for the patients in our study were 98.1±1.0% (standard error) and 91.6±2.0%, respectively. The histological subtype did not influence outcome of the patients. Comparison of patients with Ki-S5 expression (≤80% or >80%) did not reveal any significant differences in terms of survival (data not shown). When the median Ki-S2 expression (≤20% vs >20%) was used as cutoff value, there were also no differences observed. The probability of event-free survival for patients with Ki-S5 expression (≤80 or >80%) (Figure 3a) was 91.3%±2.5 vs 89.2±3.7% (P=0.41). An analysis of the impact of repp86 protein expression (≤20% or >20%) on outcome (Figure 3b) also did not show a significant difference in event-free survival (89.5±28% vs 91.7±3.4%, P=0.96). In the 19 patients with treatment failure, the proliferation rates for Ki-67 or repp86 did not differ from those in patients in first complete remission. Biopsy specimens taken at diagnosis and relapse were available only for three patients. The Ki-S5 (80%) and Ki-S2 (20%) proliferation rates did not differ between initial and subsequent investigation; however, these numbers were too small for statistical evaluation.
Also, in a multivariate analysis with the treatment group as a covariable, the proliferation rates had no significant impact on event-free survival. The risk ratio for Ki-67 staining was 0.82 (CI, 0.31–2.17, P=0.69) and for repp86 staining it was 1.11 (CI, 0.44–2.76, P=0.83).
Discussion
For the assessment of proliferation, the antigens under investigation must be restricted to proliferating cells or there must be a cell cycle-induced increase in their expression. It is known from immunohistochemical and flow cytometry studies that Hodgkin's lymphoma is characterized by high Ki-67 expression.12, 13, 15, 35, 36, 37 This finding, however, contrasts with the paucicellular nature and clinical behavior of this enigmatic lymphoma. Immunohistochemistry has an advantage over flow cytometry in that cellular morphology and histology can be more accurately interpreted. The scarcity of Hodgkin's and Reed–Sternberg cells and the high proliferation rate of bystander cells make a reliable assessment of proliferation data by means of the flow cytometry technique difficult.
A high proliferation rate was demonstrable in our series of pediatric Hodgkin's lymphoma, but it was not statistically correlated with histological subtypes, especially nodular sclerosis grade II (increased number of Hodgkin's and Reed–Sternberg cells) or advanced clinical stages. This study on Caucasian children and adolescents differs from the above cited studies in the homogeneity of its patients with respect to diagnosis and treatment. We did not find a high proliferation rate, as determined by Ki-67 antigen expression, to have a negative effect on outcome in Hodgkin's Lymphoma as suggested by other authors.35, 36, 37
Since the development of monoclonal antibodies against formalin-resistant epitopes of the Ki-67 antigen, the previously reported difficulties with poor morphology due to frozen tissues have been overcome. The available antibodies are directed against proliferation-associated antigens (eg Ki-67, PCNA and topoisomerase IIα) expressed in the G1-phase of the cell cycle. In contrast, the monoclonal antibody Ki-S2 detects a nuclear protein (repp86) that is expressed in the G2, S and M phases, but not in the G1 phase. It thus enables the interpretation of individual cell cycle phases.20 This antibody repp86 shows high sequence homology with the Xenopus spindle-associated protein TPX2 that is required for microtubule assembly and spindle pole organization.21 Repp86 expression provides more accurate evidence of proliferating Hodgkin's and Reed–Sternberg cells than Ki-67 expression in serial sections of the same diagnostic lymph node (median Ki-67: 80%, median repp86: 20%, P<0.001). The difference can be interpreted as indicating that the tumor cells are arrested in the G1 cell cycle phase, which might provide an explanation for the paradoxical finding of a high proliferation rate of Hodgkin's and Reed–Sternberg cells and lymphocytic and histiocytic cells despite slow clinical progression of the lymphoma.
Another possible explanation for the high proliferation rate in Hodgkin's lymphoma might lie in the occurrence of endomitoses, resulting in complex and variable karyotypic abnormalities.38, 39 Sequential analyses of chromosomal aberrations reveal an increasing chromosomal instability of the genome, but no arithmetic doubling of the chromosomes.40, 41 Therefore, we speculate that endomitosis does not play a central role in proliferation of Hodgkin's and Reed–Sternberg cells. If the process of endomitosis is of minor influence only, more attention should be focused on G1 arrest as a possible underlying pathogenetic mechanism.
In our study, high Ki-67 antigen expression in Hodgkin's and Reed–Sternberg cells and lymphocytic and histiocytic cells was not related to either advanced clinical stages or poor clinical outcome. The presence of B symptoms, reflecting unregulated cytokine production in Hodgkin's lymphoma,42 also did not correlate with a high proliferation rate. First-line therapy was very efficient and thus eliminated the high proliferation as a possible adverse biological factor. Our findings strongly emphasize the importance of a highly effective primary therapy in Hodgkin's lymphoma to achieve a favorable outcome in children and adolescents.
References
Hall PA, Richards MA, Gregory WM, et al. The prognostic value of Ki67 immunostaining in non-Hodgkin's lymphoma. J Pathol 1988;154:223–235.
Stokke T, Smeland EB, Kvaloy S, et al. Tumour cell proliferation, but not apoptosis, predicts survival in B-cell non-Hodgkin's lymphomas. Br J Cancer 1998;77:1839–1841.
Gerdes J, Stein H, Pileri S, et al. Prognostic relevance of tumour-cell growth fraction in malignant non-Hodgkin's lymphomas. Lancet 1987;2:448–449.
Grogan TM, Lippman SM, Spier CM, et al. Independent prognostic significance of a nuclear proliferation antigen in diffuse large cell lymphomas as determined by the monoclonal antibody Ki-67. Blood 1988;71:1157–1160.
Mochen C, Giardini R, Costa A, et al. MIB-1 and S-phase cell fraction predict survival in non-Hodgkin's lymphomas. Cell Prolif 1997;30:37–47.
Gerdes J, Schwab U, Lemke H, et al. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int J Cancer 1983;31:13–20.
Gerdes J, Dallenbach F, Lennert K, et al. Growth fractions in malignant non-Hodgkin's lymphomas (NHL) as determined in situ with the monoclonal antibody Ki-67. Hematol Oncol 1984;2:365–371.
Duchrow M, Schlüter C, Wohlenberg C, et al. Molecular characterization of the gene locus of the human cell proliferation-associated nuclear protein defined by monoclonal antibody Ki-67. Cell Prolif 1996;29:1–12.
Schmidt MH, Broll R, Bruch HP, et al. The proliferation marker pKi-67 organizes the nucleolus during the cell cycle depending on Ran and cyclin. Br J Pathol 2003;199:18–27.
Kreipe H, Wacker HH, Heidebrecht HJ, et al. Determination of the growth fraction in non-Hodgkin's lymphomas by monoclonal antibody Ki-S5 directed against a formalin-resistant epitope of the Ki-67 antigen. Am J Pathol 1993;142:1689–1694.
Cattoretti G, Becker MH, Key G, et al. Monoclonal antibodies against recombinant parts of the Ki-67 antigen (MIB 1 and MIB 3) detect proliferating cells in microwave-processed formalin-fixed paraffin sections. J Pathol 1992;168:357–363.
Gerdes J, van Baarlen J, Pileri S, et al. Tumor cell growth fraction in Hodgkin's disease. Am J Pathol 1987;128:390–393.
Claviez A, Tiemann M, Peters J, et al. The impact of EBV, proliferation rate, and Bcl-2 expression in Hodgkin's disease in childhood. Ann Hematol 1994;68:61–66.
Abele MC, Valente G, Kerim S, et al. Significance of cell proliferation index in assessing histological prognostic categories in Hodgkin's disease. An immunohistochemical study with Ki67 and MIB-1 monoclonal antibodies. Haematologica 1997;82:281–285.
Morente MM, Piris MA, Abraira V, et al. Adverse clinical outcome in Hodgkin's disease is associated with loss of retinoblastoma protein expression, high Ki67 proliferation index, and absence of Epstein–Barr virus-latent membrane protein 1 expression. Blood 1997;90:2429–2436.
Aisenberg AC . Malignant Lymphoma. Biology, Natural History, and Treatment. Lea Febiger: Malvern, PA, 1991.
Hall PA, Levison DA . Assessment of cell proliferation in histological material. J Clin Pathol 1990;43:184–192.
Garcia JF, Camacho FI, Morente M, et al. Hodgkin and Reed–Sternberg cells harbor alterations in the major tumor suppressor pathways and cell-cycle checkpoints: analyses using tissue microarrays. Blood 2003;101:681–689.
Bai M, Tsanou E, Agnantis NJ, et al. Proliferation profile of classical Hodgkin's lymphomas. Increased expression of the protein cyclin D2 in Hodgkin's and Reed–Sternberg cells. Mod Pathol 2004;17:1338–1345.
Heidebrecht HJ, Buck F, Steinmann J, et al. p100: a novel proliferation-associated nuclear protein specifically restricted to cell cycle phases S, G2, and M. Blood 1997;90:226–233.
Heidebrecht HJ, Adam-Klages S, Szczepanowski M, et al. repp86: A human protein associated in the progression of mitosis. Mol Cancer Res 2003;1:271–279.
Schellong G, Pötter R, Brämswig J, et al. High cure rates and reduced long-term toxicity in pediatric Hodgkin's disease: The German–Austrian multicenter trial DAL-HD-90. The German–Austrian Pediatric Hodgkin's Disease Study Group. J Clin Oncol 1999;17:3736–3744.
Dörffel W, Lüders H, Rühl U, et al. Preliminary results of the multicenter trial GPOH-HD 95 for the treatment of Hodgkin's disease in children and adolescents: analysis and outlook. Klin Pädiatr 2003;215:139–145.
Lukes RJ, Butler JJ . The pathology and nomenclature of Hodgkin's disease. Cancer Res 1966;26:1063–1083.
Stein H, Delsol G, Pileri S, et al. Hodgkin lymphoma In: Jaffe ES, Harris NL, Stein H, Vardiman JW (eds). World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of Hematopoietic and Lymphoid Tissues. IARC Press: Lyon, 2001, pp 237–253.
Bennett MH, MacLennan KA, Easterling MJ, et al. The prognostic significance of cellular subtypes in nodular sclerosing Hodgkin's disease: an analysis of 271 non-laparotomised cases (BNLI report no. 22). Clin Radiol 1983;34:497–501.
Shi SR, Key ME, Kalra KL . Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 1991;39:741–748.
Hsu SM, Raine L, Fanger H . Use of avidin–biotin–peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem 1981;29:577–580.
Cordell JL, Falini B, Erber WN, et al. Immunoenzymatic labeling of monoclonal antibodies using immune complexes of alkaline phosphatase and monoclonal anti-alkaline phosphatase (APAAP complexes). J Histochem Cytochem 1984;32:219–229.
Ishii Y, Takami T, Yuasa H, et al. Two distinct antigen systems in human B lymphocytes: identification of cell surface and intracellular antigens using monoclonal antibodies. Clin Exp Immunol 1984;58:183–192.
Schwarting R, Gerdes J, Dürkop H, et al. BER-H2: a new anti-Ki-1 (CD30) monoclonal antibody directed at a formol- resistant epitope. Blood 1989;74:1678–1689.
Rudolph P, Knuchel R, Endl E, et al. The immunohistochemical marker Ki-S2: cell cycle kinetics and tissue distribution of a novel proliferation-specific antigen. Mod Pathol 1998;11:450–456.
Kaplan ES, Meier P . Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958;53:457–481.
Peto R, Pike MC, Armitage P, et al. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. Analysis and examples. Br J Cancer 1977;35:1–39.
Morgan KG, Quirke P, O’Brien CJ, et al. Hodgkin's disease: a flow cytometric study. J Clin Pathol 1988;41:365–369.
Joensuu H, Klemi PJ, Korkeila E . Prognostic value of DNA ploidy and proliferative activity in Hodgkin's disease. Am J Clin Pathol 1988;90:670–673.
Erdkamp FL, Breed WP, Schouten HC, et al. DNA aneuploidy and cell proliferation in relation to histology and prognosis in patients with Hodgkin's disease. Ann Oncol 1993;4:75–80.
Drexler HG, Gignac SM, Hoffbrand AV, et al. Formation of multinucleated cells in a Hodgkin's-disease-derived cell line. Int J Cancer 1989;43:1083–1090.
Gupta RK, Lister TA, Bodmer JG . Proliferation of Reed–Sternberg cells and variants in Hodgkin's disease. Ann Oncol 1994;5 (Suppl 1):117–119.
Schlegelberger B, Weber-Matthiesen K, Himmler A, et al. Cytogenetic findings and results of combined immunophenotyping and karyotyping in Hodgkin's disease. Leukemia 1994;8:72–80.
Falzetti D, Crescenzi B, Matteuci C, et al. Genomic instability and recurrent breakpoints are main cytogenetic findings in Hodgkin's disease. Haematologica 1999;84:298–305.
Gruss HJ, Kadin ME . Pathophysiology of Hodgkin's disease: functional and molecular aspects. Baillieres Clin Haematol 1996;9:417–446.
Acknowledgements
This study was supported by the Kinderkrebs-Initiative Buchholz (Holm-Seppensen).
Author information
Authors and Affiliations
Corresponding author
Additional information
Financial disclosure
We confirm that we have no potential conflict of interest to declare.
Rights and permissions
About this article
Cite this article
Tiemann, M., Claviez, A., Lüders, H. et al. Proliferation characteristics in pediatric Hodgkin's lymphoma point to a cell cycle arrest in the G1 phase. Mod Pathol 18, 1440–1447 (2005). https://doi.org/10.1038/modpathol.3800466
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800466
Keywords
This article is cited by
-
MET and MST1R as prognostic factors for classical Hodgkin's lymphoma
Modern Pathology (2013)
-
Pediatric Lymphoma Diagnosis: Role of FNAC, Biopsy, Immunohistochemistry and Molecular Diagnostics
The Indian Journal of Pediatrics (2013)