Abstract
The proximity and, in some instances, communication between several structures in the testis and paratestis (rete testis, epididymis, mesothelium, vestigial epithelium and paratesticular soft tissue) result in a plethora of interesting tumors and tumor-like lesions that together pose a formidable diagnostic challenge both because of their morphologic overlap and rarity. The occasional spread of tumors primarily at other sites to this region adds to the potential problem encountered. This review provides an overview of the pathology of nonmesenchymal paratesticular neoplasms and pseudotumors with a focus on the approach to tubulopapillary neoplasms for which diagnostic considerations may include carcinoma of the rete testis, malignant mesothelioma, ovarian-type epithelial tumors, epididymal carcinoma and metastatic carcinomas. The cornerstone of accurate characterization of these lesions is still a comprehensive, traditional clinicopathologic approach, clinical history (of another primary), gross examination (location) and routine light microscopy, but judicious incorporation of contemporary immunohistochemical markers may aid or in some instances be crucial in resolving the problems encountered.
Similar content being viewed by others
Main
Although the great majority of testicular neoplasms are germ cell tumors and most of the remainder fall in the sex cord-stromal category, miscellaneous other neoplasms and pseudotumors of various types are encountered. They are often both morphologically intriguing and diagnostically challenging. Although some of these are strictly speaking not ‘gonadal’ inasmuch as they are paratesticular, they still occur within the scrotal sac and from the clinical viewpoint the differential diagnosis is often that of a ‘testicular’ mass. Selected lesions from within this spectrum of processes are reviewed here.
Lesions of the rete testis (Table 1)
Non-Neoplastic Rete Epithelial Proliferations
Real or apparent adenomatous hyperplasia of the rete occurs in association with various testicular abnormalities, most frequently testicular atrophy.1, 2, 3 In these cases, the appearance is probably one of relative prominence rather than a ‘true’ hyperplasia, but the distinction between hyperplasia and relative prominence is arbitrary. Bona fide hyperplasia is usually an incidental finding and is grossly manifest as a solid or cystic mass in less than half of the cases. In these cases, the separation between hyperplasia and adenoma is also debatable but again of no clinical importance.
Microscopically, adenomatous hyperplasia is characterized by a complex interconnecting proliferation of tubulopapillary channels that may be focal or diffuse and with or without cystic dilatation (Figure 1).3, 4 The lumens may be empty, sperm containing or have eosinophilic secretion (Figure 1b). The lining cells are cuboidal to low columnar with innocuous cytology (Figure 1c). Continuity with nonhyperplastic rete provides clues to a rete origin and the incidental presentation and usual background of other testicular pathology indicates a non-neoplastic nature.
Adenomatous rete testis hyperplasia has been reported in patients with cryptorchidism, various nongerm cell tumors and in patients with chronic hepatic insufficiency and bilateral renal dysplasia.5 In patients with germ cell tumors, another form of hyperplasia occurs which, in contrast to ‘adenomatous hyperplasia,’ is invariably an incidental finding and is not grossly cystic.6 In these cases, hyperplastic rete epithelium fills variably expanded channels often with intracytoplasmic eosinophilic (less than 1 μm to greater than 10 μm) hyaline globules (Figure 2). This pattern may be mistaken for yolk sac tumor, although the arborizing architecture of the rete is retained and is diagnostic of the process (Figure 2b).6 The globules most likely represent proteins absorbed by hyperplastic rete epithelium, which is stimulated by neoplastic invasion.
Rete testis hyperplasia associated with germ cell tumor. Hyperplastic rete epithelium fills expanded channels (low power) (a). Seminoma associated with rete testis hyperplasia (b). Hyperplastic rete epithelium shows intracytoplasmic and extracellular hyaline globules that result in an appearance simulating yolk sac tumor. If misinterpreted as such, this would lead a pure seminoma to be diagnosed as a mixed germ cell tumor with seminoma and yolk sac tumor components.
Benign Tumors of the Rete Testis
The rare benign tumors of the rete testis (grossly circumscribed neoplasms microscopically lined by bland cuboidal to columnar cells sometimes exhibiting transition with normal or hyperplastic epithelium) span a spectrum from solid proliferations of closely packed tubules (adenoma) to those with a conspicuous cystic component (cystadenoma), a papillary architecture (papillary cystadenoma) or a fibromatous stromal component (adenofibroma).1, 5 A distinctive variant with solid tubules resembling Sertoli cell tumor of the testicular parenchyma has been reported—sertoliform cystadenoma of the rete testis.7 These latter tumors, which are arguably the most intriguing, have ranged from microscopic to 3 cm, and have all occurred in adults. Dilated rete channels contain a proliferation of solid tubules lined by tall columnar cells with eosinophilic to pale cytoplasm and have basal nuclei with distinct nucleoli; apart from the intracystic rete aspects there is a striking resemblance to a Sertoli cell tumor (Figure 3). In cases with more prominent sclerotic stroma, tumor cells may be arranged in single file cords or thin trabeculae (Figure 3b); these are also features of some Sertoli cell tumors. One of the microscopic tumors occurred at the junction of the rete and the testicular parenchyma. This is in keeping with the putative embryologic derivation of the rete from both the sex cords and the mesonephros. Further support of this concept is provided by inhibin positivity (limited experience) (Figure 3c). Although some might prefer to simply classify these lesions as Sertoli cell tumors, the location and low power characteristics place the lesion in the family of tumors of the rete in our opinion.
Carcinoma of Rete Testis
Carcinomas of the rete testis are also rare. A theme in the recent literature on this topic is the necessity to use strict criteria, as careful scrutiny of some tumors reported as in this category shows that they are likely other lesions.8 Most of the cases were reported before ovarian-type surface epithelial tumors of the testis and paratestis were described, and many did not have an optimal immunohistochemical workup to rule out malignant mesothelioma or papillary serous carcinoma. Other cases may ultimately have been proven metastatic carcinoma with longer follow-up as the latter may closely mimic rete carcinoma. Although less than 30 have sufficient strong criteria for inclusion in this category1, 5, 8, 9, 10 the differential diagnosis is realistic in a much larger number of cases and sound knowledge of the issues related to diagnosing carcinoma of the rete are important when evaluating problematic paratesticular epithelial lesions.
The following are criteria we use for diagnosing primary carcinoma of the rete (revised from Nochomovitz and Orenstein): (1) absence of histologically similar extra-scrotal tumor that plausibly could be the primary site, (2) tumor centered on the hilum, (3) morphology incompatible with any other type of testicular or paratesticular tumor and (4) immunohistochemical exclusion of other possibilities, particularly malignant mesothelioma and papillary serous carcinoma.1, 8, 9, 11 Visualization of a transition from normal to neoplastic rete epithelium is also a useful diagnostic clue if present (Figure 4a), but large masses often obliterate the normal anatomy such that a transition is not evident. The criterion of a predominantly solid gross appearance was initially proposed to exclude cystic papillary serous neoplasms, but is not valid as cystic rete testis carcinomas occur.
Rete testis adenocarcinoma occurs most frequently in elderly males (commonly over 60 years of age), but has a wide age range of 8–91 years.1, 5, 8, 9, 10 Most patients present with scrotal pain or swelling, and up to a quarter of the tumors are associated with hydrocele. Survival is dismal, average 8 months, but long-term survival of over 4 years has been reported.
The tumors are centered in the testicular hilum, and are typically large; satellite nodules that involve the spermatic cord are seen in up to one-third of cases. Microscopically, they show a range of glandular patterns with tubulo-glandular being typical, but also including elongated and compressed branching tubules (retiform), solid tubular (sertoliform), papillary (Figure 4a–c), mainly solid with occasionally tiny slit-like channels (‘kaposiform’), and a biphasic epithelial and spindled pattern.8, 9, 10 Papillary growth is often as small glomeruloid structures within cysts. The cells are cuboidal to rarely columnar with eosinophilic cytoplasm, nuclear stratification and at least moderate pleomorphism with easily found mitoses except when they line cysts in which instance compression may result in a deceptively innocuous appearance. Necrosis is variable. The stroma may be inflammatory and desmoplastic.
The differential diagnosis includes malignant mesothelioma, certain ovarian-type tumors, metastatic adenocarcinoma, epididymal carcinoma and malignant Sertoli cell tumor.1, 2, 5, 9 Mesotheliomas may show remarkable architectural similarity to rete testis adenocarcinoma, but can be distinguished based on combined gross and routine microscopic features in most cases. Grossly malignant mesothelioma typically forms ill-defined neoplastic tissue coating or involving the tunica vaginalis in a multifocal fashion. This is in marked contrast to the discrete solitary rete-based mass of rete carcinoma. The typical tubulopapillary pattern of most mesotheliomas is in contrast to the slit-like and cellular bud-like papillary pattern (Figure 4a, c) of most rete carcinomas, but in individual cases there may be overlap such as one striking case of mesothelioma we have seen in which slit-like spaces were striking. The cytologic atypia in malignant mesothelioma is generally less striking than in cases of rete testis carcinoma. Immunohistochemistry may be a useful adjunct in particularly challenging cases, or serve a confirmatory role in more straightforward cases. Positivity for mesothelioma-related markers (calretinin, WT-1, CK 5/6) and negativity for adenocarcinoma-related antibodies (CEA, Leu-M1, Ber EP4 and B72.3) are key to confirming a mesothelioma diagnosis. Müllerian-type tumors, particularly serous and occasionally endometrioid, may mimic rete testis adenocarcinoma to a limited degree. Serous tumors of low malignant potential are typically prominently cystic but their well-known morphology differs from that of frank carcinoma, rete or otherwise, and they have blander cytology than rete carcinomas. Frequent psammoma bodies and occasional concurrent divergent differentiation, mucinous or squamous, help in the differential with frank serous carcinoma, which is typically centered in the epididymo-testicular grove. CA-125 and WT-1 are important immunomarkers to confirm Müllerian-type derivation (see the immunohistochemistry of testicular and paratesticular tumors with tubulopapillary architecture section).
Metastatic adenocarcinoma to the paratesticular soft tissue may infiltrate between and blend with the rete structures, simulating primary rete testis carcinoma. Four clues are helpful in this regard: metastatic carcinoma (1) is very commonly bilateral and/or multifocal, (2) demonstrates frequent vascular-lymphatic invasion, (3) has an interstitial pattern of growth (in between rather than from rete epithelium) and (4) usually occurs in patients with a history of a primary carcinoma, although sometimes the history is remote.
The vanishingly rare adenocarcinoma of the epididymis may be solid, widely destructive of the epididymis and infiltrative into paratesticular tissue or, less commonly, be a localized cystic mass.12 Microscopically, these tumors have predominately a tubular pattern but cystic dilatation may produce a tubulocystic appearance, and papillae a tubulopapillary one. Nonspecific foci of solid growth may also be seen. The cells are typically cuboidal to columnar cells with appreciable to abundant cytoplasm that may be clear, amphophilic or eosinophilic. Some epididymal carcinomas have only mild to moderate nuclear atypicality with low mitotic activity in contrast to rete testis carcinoma. Prominent clear cells in a tubular carcinoma of this region favor epididymal carcinoma.
Malignant Sertoli cell tumors and rete testis adenocarcinoma may be confused because the latter may have sertoliform tubules. Sertoli cell tumors are distinguished by their predominant location within the testicular parenchyma rather than the rete. This is one of many examples of the crucial importance of gross evaluation in the differential diagnosis of testicular and paratesticular neoplasms. Sertoliform rete carcinomas may have foci of more typical rete carcinoma.
Ovarian-Type Epithelial Tumors
These tumors are testicular, or more commonly, paratesticular homologues of their more common ovarian counterparts. The entire spectrum of histologic cell types seen in the ovary has been described in the following approximate order of frequency: serous, mucinous, endometrioid, clear cell, transitional (Brenner) and exceptionally squamous (Table 2).13, 14, 15, 16, 17, 18 Serous and mucinous tumors, mostly of borderline type, are much more frequent than other subtypes but are still rare, with less than 40 reported serous tumors and approximately 15 reported mucinous tumors.9, 13, 14, 15 Given the histologic identity with ovarian counterparts, most studies have applied the criteria and nomenclature of the ovarian tumors.
The histogenesis of ovarian-type epithelial tumors of the testis and paratestis remains speculative with favored theories for most being Müllerian metaplasia of the tunica vaginalis and origin from Müllerian rests in paratesticular soft tissue or the appendix testis (based on many tumors being centered on the epididymo-testicular groove).8, 16 Intratesticular tumors may develop from mesothelial inclusions or, particularly in the case of mucinous tumors, represent monodermal teratomas; if the latter, the absence of associated intratubular germ cell neoplasia suggests a pathogenetic paradigm that is different from most testicular germ cell tumors and perhaps similar to that suggested for testicular dermoid cysts.15 Brenner tumors may arise from the relatively common Walthard rests of the tunica (Figure 5).
Serous Neoplasms
The mean age for borderline tumors is 56 years (range: 14–77 years) and for invasive tumors, 31 years (range: 16–42 years).13, 14, 16 Dull pain, swelling, palpable mass and associated hydrocele are presenting signs and symptoms. CA-125 levels may be elevated. Borderline tumors are invariably cystic with a thin fibrous capsule that is usually contiguous with the tunica vaginalis (Figure 6a). Carcinomas are usually noncystic and infiltrative.
Microscopically, borderline tumors exhibit papillae with fibrovascular cores lined by stratified cuboidal to columnar epithelium ranging from bland to atypical with variable mitotic activity (Figure 6b). Frequent psammoma bodies, ciliated and hobnail cells are clues to serous differentiation.1, 13, 14, 16 Microinvasion (using ovarian criteria) may infrequently be seen in borderline tumors, however, one such case has been clinically malignant, indicating that ovarian criteria may not be appropriate in this region and further experience with this form of serous neoplasia, and for that matter other forms, is required before any firm conclusions concerning prognostic criteria can be made.19 Generous sampling and continued clinical surveillance are thus required for all borderline tumors. The prognosis is usually excellent for completely excised borderline serous tumors.13 Papillary serous carcinomas are capable of recurrence and distant metastasis.12 Invasive tumors usually have well-formed papillary structures associated with a desmoplastic stroma and frequent psammoma bodies (Figure 6c). An associated borderline tumor component may help to place a tumor in the papillary serous carcinoma category.1
Mucinous Tumors
The mean age of reported cases is 52 years (range: 11–69 years).15 Interestingly, a subset of tumors present as intratesticular masses and mucinous cystadenoma has been reported (in contrast to serous tumors, which are almost always paratesticular, with no reported cases of cystadenoma). Borderline tumors are predominantly cystic (mean size=3.5 cm) and contain copious amounts of gelatinous material, whereas mucinous carcinoma is frequently solid, infiltrative and may cause nodular thickening of the tunica vaginalis.
Microscopically, cystadenomas are lined by tall columnar endocervical-like cells with basally situated nuclei without atypia (Figure 7a).1, 15, 16 Mucinous cystic tumors of borderline malignancy and carcinomas are typically lined by intestinal-type cells. Borderline neoplasms may show intracystic complex papillary and cribriform arrangements (Figure 7b), infrequently with highly atypical cells (intraepithelial carcinoma) or limited invasion (designated microinvasive if less than 3 mm). Mucin extravasation, chronic inflammation, calcification and ossification may be present in adjacent testicular and paratesticular tissue. The differential diagnosis for intratesticular mucinous tumors most realistically includes teratoma. Features helpful in excluding teratoma include absence of other teratomatous or germ cell tumor components and absence of intratubular germ cell neoplasia. Additionally, teratomas frequently occur in the age group of 18–30 years, whereas mucinous tumors, in the only reported series, occurred in patients 44–69 years of age (mean=64 years). Metastatic adenocarcinoma and extension of a low-grade mucinous tumor from the appendix along the peritoneal surfaces into an inguinal hernia sac are differential diagnostic possibilities for paratesticular mucinous tumors.15 The clinical history of a primary carcinoma elsewhere, bilaterality, interstitial growth and frequent vascular invasion favor metastatic carcinoma. Mucinous tumors of the appendix that involve the inguinal region of men usually involve a hernia sac and have a more prominent extracellular mucinous component with absent or only small cysts.20 Appendiceal and metastatic colonic tumors are frequently CK7 negative in contrast to most primary ovarian-type mucinous tumors, which are CK7 positive.
The outcome of cystadenomas and tumors of borderline malignancy (including those with intraepithelial carcinoma or microinvasion) has been benign (follow-up of up to 12 years, median 2 years).15 Mucinous carcinoma has the potential for intra-abdominal peritoneal spread (reported in one case with fatal outcome), although the only other case with follow-up greater than 2 years (14 years) had no evidence of disease progression.21
Mesothelial lesions
Reactive Mesothelial Hyperplasia
Persistent serosal injury, as in hydrocele, hematocele, inguinal hernia sacs and miscellaneous other settings may cause reactive hyperplasia of the mesothelial lining and submesothelial fibrosis.1, 22, 23, 24 The combination of the two responses may easily result in histology mimicking malignant mesothelioma, papillae and small tubules, solid nests and cords extending into the underlying reactive connective tissue simulating invasion (Figure 8a, b). Small reactive mesothelial tubules entrapped within fibrous tissue, with retraction artifact, may mimic adenocarcinoma with vascular invasion (Figure 8b). Further, reactive atypia of mesothelial cells produces a cytologic picture overlapping with that of malignant mesothelial cells that are often not particularly malignant-appearing cytologically.22, 24 Architectural features, complex tubulopapillary proliferations with extensive destructive and confluent infiltration, and marked cytologic atypia both point to malignancy. A gross mass rules out mesothelial hyperplasia but its absence does not exclude mesothelioma. Desmin has recently been shown to have value in this differential as it is infrequently expressed in malignant mesothelioma and commonly positive in hyperplasia.25 Metastatic adenocarcinoma is a rare differential diagnostic consideration. Low-power observation of a linear disposition of the tubules rather than a haphazard infiltrative growth, reactive background such as of an organizing hematocele (Figure 8a) and positivity for mesothelial markers confirm reactive mesothelial hyperplasia (Figure 8c) rather than metastatic adenocarcinoma.1, 22, 24
Reactive mesothelial hyperplasia with mesothelial entrapment occurring in a background of organizing hematocele. Low-power observation of linear disposition of tubules rather than a haphazard infiltrative growth is an important feature to recognize (a). Higher power shows small reactive tubulopapillary mesothelial clusters with retraction artifact that may mimic vascular invasion (b). Immunohistochemical stain for WT1 confirms the mesothelial nature of the proliferation (c).
Adenomatoid Tumor
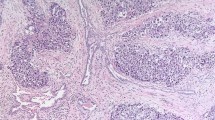
This is a distinctive common benign mesothelial neoplasm of the paratesticular region, most commonly occurring at the head of the epididymis but often within the tunica albuginea with secondary involvement of the adjacent testicular parenchyma. Gross examination usually makes it clear that the neoplasm is paratesticular or juxtatesticular but rarely parenchymal involvement is so extensive that the gross impression is that of a bone fide testicular neoplasm. Their size ranges up to about 7 cm with the majority being in the range of 3–5 cm. The tumor is invariably well circumscribed grossly and varies from being oval to crescent-shaped. It is typically white and, although generally firmer than the usual seminoma, cases with parenchymal involvement of note may be grossly indistinguishable from seminoma. The tumor has a plethora of microscopic appearances represented by three basis patterns: tubules, cords, and small nests lined by or formed of cells that are cuboidal with moderate to occasionally abundant amphophilic, eosinophilic (Figure 9) or vacuolated cytoplasm.1, 2, 24 The vacuoles represented by the latter represent an important albeit sometimes subtle clue to the diagnosis. Relatively common features complicate the basic pattern of tubules, cords and nests. First of all, these three components are often so closely packed that a more or less solid pattern is evident, at least on low power. A ‘true’ solid or diffuse pattern is rare. Secondly, the tubules are often markedly dilated and irregular in size and shape, producing a striking cystic morphology on low power. Thirdly, the stroma may be prominent and an irregular disposition of the tubules, cords and nests within it may suggest infiltration and bring into consideration diverse entities such as malignant mesothelioma and secondary carcinoma. The stroma, which in most cases is nonetheless minor, is typically fibrous and occasionally hyalinized. Finally, adenomatoid tumor, although grossly circumscribed, often infiltrates microscopically between the testicular tubules, a finding that should not be misconstrued as evidence of malignancy. A helpful low-power clue to the diagnosis of adenomatoid tumor in many cases is the presence of prominent lymphoid aggregates, particularly towards the periphery of the neoplasm (Figure 9a). These are rarely seen in other lesions in the differential. Sometimes thread-like bridging strands may traverse tubular or pseudoglandular spaces, another finding essentially specific to this neoplasm.26 Owing to the above range of appearance, adenomatoid tumor may pose a spectrum of potential diagnostic problems, as summarized in Table 3 (Figure 9b).
Adenomatoid tumor. Low power shows cords of eosinophilic cells embedded within a fibrous stroma containing lymphoid aggregates (a). High power of a more cellular area with abundant eosinophilic cytoplasm arranged in cords and trabeculae. This histology may raise the possibility of a Leydig cell tumor of testis (b). Infarcted adenomatoid tumor with gaping spaces with ghost outlines of necrotic cells (c). The edge of the infarcted tumor may show viable cells displaying greater atypia which, when associated with a florid reactive fibroblastic and myofibroblastic stroma, may result in an alarming histology (d).
Yet another aspect of the morphology of adenomatoid tumors has only relatively been highlighted and is now discussed in more detail. Infarction of adenomatoid tumors may obscure the nature of the underlying lesion, and a surrounding florid reactive fibroblastic and myofibroblastic proliferation blurs the boundary with adjacent tissue, with infiltration of fat by reactive tissue or tumor cells (Figure 9c, d). This represents, in aggregate, an important diagnostic pitfall.27 A solid architecture in viable areas and reactive atypia can be further alarming. The infarction may result in pain, which is an unusual presentation for an adenomatoid tumor. Simple awareness of this phenomenon is crucial to the correct diagnosis, which should be entertained when evaluating a necrotic mass of the paratesticular region. As in resolving the nature of any necrotic genital tract lesion, careful microscopy usually discloses clues that at first impression may not be obvious. Gaping spaces with no evident lining, representing a necrotic tubular component, and smaller spaces, representing ghost remnants of the typical vacuolar spaces, are major clues to the diagnosis.
In virtually all of the differential diagnostic considerations, appreciating the overall cardinal features of adenomatoid tumors is essential to the correct diagnosis. This includes the usually asymptomatic presentation, the unilateral, solitary, well-demarcated appearance of the lesion, and the range of patterns and stromal features. One of the most realistic problems, albeit one with little or no clinical import, is the differential with a Sertoli cell tumor, because the tubules of these tumors can be very similar.28 Additionally, the vacuoles of adenomatoid tumor can be simulated by lipidized Sertoli cells to an extent. The basophilic mucin in the tubules of some adenomatoid tumors may be helpful. Immunohistochemical confirmation with mesothelial-related markers (calretinin, CK5/6 and WT-1) is helpful in the differential with nonmesothelial lesions, even in the infarcted tumors.27, 29 Electron microscopy will also help for noninfarcted lesions, but is rarely necessary.
Malignant Mesothelioma
This is the most common malignant paratesticular tumor with an ‘epithelial’ growth pattern and invariably enters the differential diagnosis of a tubulopapillary neoplasm at this site.2, 8, 24, 30, 31 It occurs most commonly in men in the sixth or seventh decade, although it also occurs rarely in younger patients, including children. Patients present with unilateral testicular swelling or recurrent hydrocele. Only rarely is the presentation incidental in a hernia sac or is there a palpable mass clinically suggestive of a neoplasm. Asbestos exposure remains the only known risk factor and is documented in more than one-third of the cases; information on exposure, however, may not always have been adequate.1, 24, 30, 31
Although there are reports of small (less than 2 cm), asymptomatic, solitary lesions of low nuclear grade and lacking mitotic activity (described in the literature as ‘well-differentiated papillary mesothelioma’)32 and large multicystic tumors lined by flattened to low cuboidal mesothelial cells lacking cytologic atypia (described as ‘benign cystic mesothelioma’),33 prolonged follow-up of appreciable numbers of tumors showing a benign course is unavailable, and some have exhibited progressive disease, supporting classification in the broad malignant mesothelioma category.1 Localized tumors with the above features may be flagged as having potentially favorable outcomes if completely excised but deserve the same meticulous follow-up as conventional diffuse malignant mesothelioma. These comments notwithstanding, we have seen one convincing example of peritoneal inclusion cysts, a diagnosis that should be reserved for cases with the typical morphology seen in females and the usual inflammatory background. The prognosis for patients with malignant mesothelioma overall is guarded, with 45% of the patients dying within 2 years.30, 31 While most recur early, recurrence after 10 years has been documented such that long-term follow-up is necessary.24, 30, 31
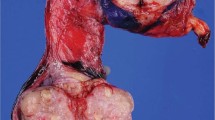
Gross examination reveals various appearances ranging from diffuse thickening of the tunica vaginalis or multiple nodules on it, sometimes coating it (Figure 10a, b). Infiltration of the testis, epididymis or cord structures is frequent. Microscopically, the tumors resemble their pleural and intra-abdominal peritoneal counterparts. Pure epithelial (60–70%) (Figure 10c) or biphasic (30–40%) (Figure 10d) histology is most frequently observed, but very rarely pure sarcomatous histology, including the desmoplastic variant, may be seen (Figure 10e).1, 24, 30 The entire spectrum of differentiation from well-differentiated tumors (tubulopapillary architecture, simple to typically more complex arborizing fibrovascular cores lined by a single layer or multilayered mildly atypical mesothelial cells, and variably invasive tubules) to poorly differentiated tumors (solid sheets, cords and nests of highly infiltrative epithelioid cells with necrosis) may be seen. Foam cells, psammoma bodies and wide variation in glandular patterns (small tubules, irregular slit-like anastomosing channels and large glands and cysts with intracystic proliferation) may be present, compounding the histologic overlap with several other tumors that occur in the region. Focal areas may resemble an adenomatoid tumor. Careful gross examination, targeted sampling of tumor/tunica vaginalis interface and extensive sectioning may reveal transitions from normal to hyperplasia to invasive malignancy (Figure 10c). The tumor may invade paratesticular soft tissue, skin of the penis, scrotum or the testis, where it may occasionally have an intratubular pattern of growth. The differential diagnosis includes, at the well-differentiated end, mesothelial hyperplasia (discussed previously) and tumors with glandular architecture (discussed in the differential diagnosis of rete testis adenocarcinoma and summarized in Table 4). Tumors with spindle cell morphology may mimic sarcomas, and those composed of solid sheets of epithelioid cells may have a broad differential diagnosis with a number of malignant epithelial tumors and metastatic malignant melanoma. Immunohistochemistry, and even occasionally electron microscopy, may play a major role in the spindle cell and extensively epithelioid tumors, but thorough sampling is even more important.
Malignant mesothelioma of paratestis. Extensive nodular tumefaction of entire tunica vaginalis (a). Diffuse thickening of the tunica vaginalis with multiple nodules (b). Pure epithelial histology. Transition of tumor from more normal appearing tunica vaginalis is a useful diagnostic clue (c). Malignant mesothelioma with biphasic histology (d). Desmoplastic variant of malignant mesothelioma (e). (Figure b and e courtesy Dr Robert H Young, Boston, MA, USA.)
Secondary tumors
Hematopoietic tumors of all major types may secondarily involve the testis, and the diagnosis of lymphoma in particular should always be kept in mind, particularly in older men, assuming the morphology is in any way compatible with the diagnosis.34, 35, 36 From the gross viewpoint, the noteworthy issue is the extent to which lymphoma may simulate seminoma, although it is helpful that lymphoma involvement is more often bilateral and more frequently exhibits extratesticular spread. On microscopic examination, interstitial growth with sparing of the seminiferous tubules is typically striking, certainly compared to most germ cell tumors. However, effacement of the tubules and intratubular growth may be seen and result in diverse diagnostic problems. As these and other clinical, gross and microscopic features of hematopoietic malignancies have recently been comprehensively studied by Ferry and co-workers16, 34, 35, 36, 37 in a series of original papers and in a comprehensive review, their features will not be reiterated in detail here. Cytologic characteristics and immunohistochemical profile largely determine the classification and subclassification of hematopoietic malignancies according to modern terminology as elsewhere. Although interstitial spread of lymphomas is typical, they can exhibit intratubular growth that may be confusing, as in the case of one anaplastic large cell lymphoma with central necrosis that simulated intratubular embryonal carcinoma.37 Confusion may be further heightened by the expression of CD30, but a comprehensive panel of other markers will assist, provided the pitfall is known. The rest of this discussion of secondary tumors focuses on the spread of solid tumors, if only because this problem has not received the same degree of recent consideration as lymphoma and related lesions and, furthermore, the topic has received much less attention than the comparable problem in the female gonad. Finally, our experience indicates that a diagnostically interesting and challenging array of issues may arise.
Although occasional spread to the testis is secondary, from adjacent sites such as in cases of malignant mesothelioma, this is an issue unto itself considered earlier and the focus here is on metastasis from distant sites. Spread to the paratestis is also considered, as the clinical differential is often that of a true testicular tumor.
In one series, 3.6% of testicular tumors were metastatic and, in another, 8.1% of malignant tumors of the paratestis (Figure 11) were metastatic.38, 39 Metastasis to the testis most commonly is from the prostate (33%) or lung (20%),5, 8 with other sources being cutaneous malignant melanoma (8%), appendix and colorectal malignancy (8%), kidney (6%), stomach, pancreas and carcinoid tumors and other rarer sites.5, 1, 40, 41 Metastasis to the paratestis occurs more commonly from carcinomas of the stomach (43%) and the prostate (29%).1, 5, 42 Other primary sites are similar to those of metastasis to the testis. Features pointing to the possibility of metastatic tumor include (1) age greater than 50 years, (2) history of a primary tumor at another site, (3) bilateral or multifocal tumors, (4) histology not resembling a known, or at least common, primary testicular or paratesticular tumor, (5) distinctive histology suggestive of a particular nonparatesticular primary malignancy, (6) frequent vascular-lymphatic invasion and (7) interstitial pattern of growth.1, 5 Depending on the histology of the metastatic tumor, various primary testicular and paratesticular tumors may be mimicked. A selected listing is provided in Table 5.
Immunohistochemistry of testicular and paratesticular tumors with tubulopapillary architecture
Although the cornerstone to arriving at the correct diagnosis is careful gross evaluation (epicenter of mass), routine light microscopy and consideration of clinical findings to rule out metastasis, several relatively sensitive and specific immunomarkers have been developed in recent years that may potentially be very useful aids to the differential diagnosis. These include mesothelioma-related markers (calretinin, WT-1, thrombomodulin, CK 5/6),11, 43 adenocarcinoma-related markers (CEA, Leu M1, B72.3, Ber EP4),43 Wolffian-derived structures-associated markers (CD10, calretinin), Müllerian papillary serous neoplasm-associated markers (WT-1, CA125); a sex-cord tumor-associated marker (inhibin), and markers useful in characterizing metastasis of unknown origin (prostate-specific antigen, prostate-specific acid phosphatase, CDX-2, TTF-1 and CK7/CK20 coexpression pattern).44, 45, 46, 47, 48 Obviously, a judicious selection from the above comprehensive list is required based on the most likely differential diagnoses. Selected panels are summarized in Table 6. A systematic and algorithmic approach combining the use of clinical information, macroscopic and microscopic examination and immunohistochemistry is outlined in Table 7.
References
Ulbright TM, Amin MB, Young RH . Tumors of the Testis, Adnexa, Spermatic Cord, and Scrotum, 3rd Series, Fascicle 25. Armed Forces Institute of Pathology: Washington, DC, 1999.
Srigley JR, Hartwick RW . Tumors and cysts of the paratesticular region. Pathol Annu 1990;25 (Part 2):51–108.
Hartwick RW, Ro JY, Srigley JR, et al. Adenomatous hyperplasia of the rete testis. A clinicopathologic study of nine cases. Am J Surg Pathol 1991;15:350–357.
Nistal M, Castillo MC, Regadera J, et al. Adenomatous hyperplasia of the rete testis. A review and report of new cases. Histol Histopathol 2003;18:741–752.
Jones EC, Murray SK, Young RH . Cysts and epithelial proliferations of the testicular collecting system (including rete testis). Semin Diagn Pathol 2000;17:270–293.
Ulbright TM, Gersell DJ . Rete testis hyperplasia with hyaline globule formation. A lesion simulating yolk sac tumor. Am J Surg Pathol 1991;15:66–74.
Jones MA, Young RH . Sertoliform rete cystadenoma: a report of two cases. J Urol Pathol 1997;7:47–53.
Nochomovitz LE, Orenstein JM . Adenocarcinoma of the rete testis: consolidation and analysis of 31 reported cases with a review of the literature. J Urol Pathol 1994;2:1–37.
Orozco RE, Murphy WM . Carcinoma of the rete testis: case report and review of the literature. J Urol 1993;150:974–977.
Menon PK, Vasudevarao, Sabhiki A, et al. A case of carcinoma rete testis: histomorphological, immunohistochemical and ultrastructural findings and review of literature. Indian J Cancer 2002;39:106–111.
Amin MB, Ulbright TM, Mendrinos SE, et al. Utility of a comprehensive immunohistochemical panel in the differential diagnosis of paratesticular neoplasms with tubulopapillary/glandular architecture. Mod Pathol 2004;17:137A.
Jones MA, Young RH, Scully RE . Adenocarcinoma of the epididymis. A report of four cases and review of the literature. Am J Surg Pathol 1997;21:1474–1480.
McClure RF, Keeney GL, Sebo TJ, et al. Serous borderline tumor of the paratestis. Am J Surg Pathol 2001;25:373–378.
Jones M, Young RH, Srigley JR, et al. Paratesticular serous papillary carcinoma. A report of six cases. Am J Surg Pathol 1995;19:1359–1366.
Ulbright TM, Young RH . Primary mucinous tumors of the testis and paratestis. A report of nine cases. Am J Surg Pathol 2003;27:1221–1228.
Henley JD, Ferry J, Ulbright TM . Miscellaneous rare paratesticular tumors. Semin Diagn Pathol 2000;17:319–339.
Goldman RL . A Brenner tumor of the testis. Cancer 1970;26:853–865.
Young RH, Scully RE . Testicular and paratesticular tumors and tumor-like lesions of ovarian common epithelial and mullerian types. A report of four cases and review of the literature. Am J Clin Pathol 1986;86:146–152.
Remmele W, Kaiserling E, Zerban U, et al. Serous papillary cystic tumor of borderline malignancy with focal carcinoma arising in testis: case report with immunohistochemical and ultrastructural observations. Hum Pathol 1992;23:75–79.
Young RH, Rosenberg AE, Clement PB . Mucin deposits within inguinal hernia sacs: a presenting finding of low-grade mucinous cystic tumors of the appendix. A report of two cases and a review of the literature. Mod Pathol 1997;10:1228–1232.
Kellert E . An ovarian type pseudomucinous cystadenoma in the scrotum. Cancer 1959;12:187–190.
Rosai J, Dehner LP . Nodular mesothelial hyperplasia in hernia sacs. A benign reactive condition simulating a neoplastic process. Cancer 1975;35:165–175.
Churg A, Colby TV, Cagle P, et al. The separation of benign and malignant mesothelial proliferations. Am J Surg Pathol 2000;24:1183–1200.
Perez-Ordonez B, Srigley JR . Mesothelial lesions of the paratesticular region. Semin Diagn Pathol 2000;17:294–306.
Attanoos RL, Griffin A, Gibbs AR . The use of immunohistochemistry in distinguishing reactive from neoplastic mesothelium. A novel use for desmin and comparative evaluation with epithelial membrane antigen, p53, platelet-derived growth factor-receptor, P-glycoprotein and Bcl-2. Histopathology 2003;43:231–238.
Hes O, Perez-Montiel DM, Alvarado Cabrero I, et al. Thread-like bridging strands: a morphologic feature present in all adenomatoid tumors. Ann Diagn Pathol 2003;7:273–277.
Skinnider BF, Young RH . Infarcted adenomatoid tumor: a report of five cases of a facet of a benign neoplasm that may cause diagnostic difficulty. Am J Surg Pathol 2004;28:77–83.
Miller F, Lieberman MK . Local invasion in adenomatoid tumors. Cancer 1968;21:933–938.
Delahunt B, Eble JN, Nacey JN, et al. Immunohistochemical evidence for mesothelial origin of paratesticular adenomatoid tumour. Histopathology 2001;38:479.
Jones MA, Young RH, Scully RE . Malignant mesothelioma of the tunica vaginalis: a clinicopathologic analysis of 11 cases with review of the literature. Am J Surg Pathol 1995;19:815–825.
Plas E, Riedl CR, Pfluger H . Malignant mesothelioma of the tunica vaginalis testis: review of the literature and assessment of prognostic parameters. Cancer 1998;83:2437–2446.
Butnor KJ, Sporn TA, Hammar SP, et al. Well-differentiated papillary mesothelioma. Am J Surg Pathol 2001;25:1304–1309.
Lane TM, Wilde M, Schofield J, et al. Benign cystic mesothelioma of the tunica vaginalis. Br J Urol Int 1999;84:533–534.
Ferry JA, Harris NL, Young RH, et al. Malignant lymphoma of the testis, epididymis, and spermatic cord: a clinicopathological study of 69 cases with immunophenotypic analysis. Am J Surg Pathol 1994;18:376–390.
Ferry JA, Srigley JR, Young RH . Granulocytic sarcoma of the testis. A report of two cases of a neoplasm prone to misinterpretation. Mod Pathol 1997;10:320–325.
Ferry JA, Young RH, Scully RE . Testicular and epididymal plasmacytoma: a report of 7 cases, including three that were the initial manifestation of plasma cell myeloma. Am J Surg Pathol 1997;21:590–598.
Ferry JA, Ulbright TM, Young RH . Anaplastic large cell lymphoma of the testis: a lesion that may be confused with embryonal carcinoma. J Urol Pathol 1996;5:139–147.
Patel SR, Richardson RL, Kvols L . Metastatic cancer to the testes: a report of 20 cases and review of the literature. J Urol 1989;142:1003–1005.
Algaba F, Santaularia JM, Villavicencio H . Metastatic tumor of the epididymis and spermatic cord. Eur Urol 1983;9:56–59.
Datta MW, Young RH . Malignant melanoma metastatic to the testis: a report of three cases with clinically significant manifestations. Int J Surg Pathol 2000;8:49–57.
Datta MW, Ulbright TM, Young RH . Renal cell carcinoma metastatic to the testis and its adnexa: a report of five cases including three that accounted for the initial clinical presentation. Int J Surg Pathol 2001;9:49–56.
Kanomata N, Eble JN . Adenocarcinoma of the pancreas presenting as an epididymal mass. A case report and literature review. J Urol Pathol 1997;6:159–170.
Ordonez NG . The immunohistochemical diagnosis of mesothelioma: a comparative study of epithelioid mesothelioma and lung adenocarcinoma. Am J Surg Pathol 2003;27:1031–1051.
Cerilli LA, Sotelo-Avila C, Mills SE . Glandular inclusions in inguinal hernia sacs: morphologic and immunohistochemical distinction from epididymis and vas deferens. Am J Surg Pathol 2003;27:469–476.
Goldstein NS, Uzieblo A . WT1 immunoreactivity in uterine papillary serous carcinomas is different from ovarian serous carcinomas. Am J Clin Pathol 2002;117:541–545.
Attanoos RL, Webb R, Dojcinov SD, et al. Value of mesothelial and epithelial antibodies in distinguishing diffuse peritoneal mesothelioma in females from serous papillary carcinoma of the ovary and peritoneum. Histopathology 2002;40:237–244.
Moskaluk CA, Zhang H, Powell SM, et al. CDX2 protein expression in normal and malignant human tissues: an immunohistochemical survey using tissue microarrays. Mod Pathol 2003;16:913–919.
Yatabe Y, Mitsudomi T, Takahashi T . TTF-1 expression in pulmonary adenocarcinomas. Am J Surg Pathol 2002;26:767–773.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amin, M. Selected other problematic testicular and paratesticular lesions: rete testis neoplasms and pseudotumors, mesothelial lesions and secondary tumors. Mod Pathol 18 (Suppl 2), S131–S145 (2005). https://doi.org/10.1038/modpathol.3800314
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800314
Keywords
This article is cited by
-
Neue WHO-Klassifikation der Hodentumoren 2022
Die Pathologie (2022)
-
Asbestos exposure and malignant mesothelioma of the tunica vaginalis testis: a systematic review and the experience of the Apulia (southern Italy) mesothelioma register
Environmental Health (2019)
-
Precocious Paratesticular Metastasis From Well-Differentiated Neuroendocrine Tumour of Ileocaecal Junction: A Case Report and Review
Indian Journal of Surgical Oncology (2017)