Abstract
Papillary thyroid carcinoma may encompass a mixed group of neoplasms where divergence in clinical behavior may reflect distinct genetic alterations. For example, young patients with papillary thyroid carcinoma have a better prognosis than affected adults, and their carcinomas are much more likely to harbor chromosomal rearrangements involving the RET proto-oncogene. Mutational activation of the BRAF oncogene has recently been identified as the most common genetic alteration in papillary thyroid carcinoma, but little is known about its frequency as a function of patient age. We tested 20 papillary thyroid carcinomas from young patients ranging from 10 to 17 years of age for the thymine (T) → adenine (A) missense mutation at nucleotide 1796 in the BRAF gene using a newly developed assay that employs a novel primer extension method (Mutector® assay). The prevalence of BRAF mutation was compared with a larger group of papillary thyroid carcinomas from previously tested adult patients (>20 years). BRAF mutations were not common in papillary thyroid carcinomas from young patients compared to their counterparts in adults (20 vs 77%; OR=13.3, 95% confidence interval (CI)=3.4–56.5; P<0.0001), but they become increasingly prevalent with advancing patient age (OR as a function of age at 10-year intervals=1.80 CI=1.33–2.44; P<0.001). Unlike papillary thyroid carcinomas that arise in adults, mutational activation of BRAF is not a major genetic alteration in papillary thyroid carcinomas that arise in young patients. The increasing frequency of BRAF mutations as a function of age could help account for the well documented but poorly understood observation that age is a relevant prognostic indicator for patients with papillary thyroid carcinoma.
Similar content being viewed by others
Main
Papillary thyroid carcinoma is a malignant tumor derived from the thyroid follicular epithelium and characterized by the formation of papillae and/or a distinctive set of nuclear features.1 However, this seemingly straightforward definition belies the diversity of the tumors it embraces. Careful microscopic analysis has resolved a number of phenotypic variants, and these subtypes of papillary thyroid carcinoma are often associated with distinct patterns of clinical behavior.2, 3 Sometimes the behavior of papillary thyroid carcinoma diverges across patient populations in ways that cannot be predicted on the basis of any microscopic features. Most notably, papillary thyroid carcinoma in young patients is associated with a better prognosis than its counterpart in adults even though it is associated with a greater risk of nodal metastases.4 Even in adults with papillary thyroid carcinoma, the fatality rate continues to increase with successive decades of life.1, 5
Differences in the behavior of papillary thyroid carcinoma across patient generations may in part reflect the accumulation of distinct genetic alterations. Fusion of the tyrosine kinase domain of the RET gene to the 5′ regulatory components of other ubiquitously expressed genes is the most common genetic alteration observed in young patients who developed papillary thyroid carcinoma following the Chernobyl reactor accident. Although the predominance of RET/PTC rearrangements has been attributed to radiation and its propensity to induce double-strand breaks, an equally high frequency of RET/PTC rearrangements has been observed in childhood papillary thyroid carcinomas unassociated with radiation exposure.6, 7, 8 Thus, translocational activation of RET may be more a function of age than exposure. In adults with sporadic papillary thyroid carcinoma, RET/PTC rearrangements are not common. Instead, the most frequent genetic alteration is mutational activation of the BRAF oncogene.9, 10, 11, 12, 13 The RET and BRAF oncogenes represent tandem signaling effectors along the mitogen-activated protein kinase pathway. Activation of RET and BRAF induces constitutive activation of the mitogen-activated protein kinase pathway, but their individual roles in thyroid tumorigenesis are mutually exclusive, not cooperative: A papillary thyroid carcinoma usually harbors a RET/PTC translocation or a BRAF mutation, but not both.11, 13 Consequently, a low prevalence of BRAF mutations would be anticipated in any form of papillary thyroid carcinoma that utilized RET/PTC as the principal mechanism of mitogen-activated protein kinase pathway activation. Given the disproportionately high rates of RET/PTC rearrangements in childhood papillary thyroid carcinoma, we tested the hypothesis that BRAF mutations vary inversely with age for patients with conventional papillary thyroid carcinoma.
Patients and methods
Sample Selection and DNA Isolation
Patients 18 years or younger with papillary thyroid carcinoma were identified from a search of the archival surgical pathology files of The Johns Hopkins Hospital between 1985 and 2004. After initial patient identification, all original histologic slides were reviewed by one of us (WHW) to confirm the diagnosis and to select an appropriate block for DNA extraction. Only those cases of conventional papillary thyroid carcinoma were included in this study. We and others have found that the BRAF mutations are absent or occur at a very low rate in the follicular variant of papillary thyroid carcinoma.10, 14, 15, 16 The paraffin blocks were sectioned, and tissues sections were microdissected to obtain greater than 80% neoplastic cells. DNA was extracted using standard protocols as previously published.17 BRAF status of papillary thyroid carcinomas in this young group of patients were compared with 65 adult patients with sporadic conventional papillary thyroid carcinomas previously evaluated using identical detection methodology.9, 10
Detection of BRAF Mutations
All tumor samples and controls were analyzed for the thymine (T) → adenine (A) missense mutation at nucleotide 1796 in the BRAF gene. This hot spot was chosen because the reported BRAF-activating mutations in thyroid carcinomas occur almost exclusively at this position.11, 12, 14 PCR primer sequences were designed to amplify a 102 bp fragment of exon 15 (5′-GAA GAC CTC ACA GTA AAA ATA GGT GA-3′, and 5′-CCA CAA AAT GGA TCC AGA CA-3′). PCR amplification was performed using 100 ng of tumor sample DNA as template. The PCR reactions were carried out in a 96-well thermocycler. Cycling conditions were as follows: A denaturation step at 95°C for 5 min was followed by two cycles of denaturation at 95°C for 1 min, annealing at 60°C for 1 min, primer extension at 72°C for 1 min, two cycles of denaturation at 95°C for 1 min, annealing at 58°C for 1 min, primer extension at 72°C for 1 min, 35 cycles of denaturation at 95°C for 1 min, annealing at 56°C for 1 min, primer extension at 72°C for 1 min, and one final extension at 72°C for 5 min. Amplified fragments were separated on an agarose gel and visualized by ethidium bromide staining.
Analysis of the PCR products for a BRAF mutation at nucleotide position 1796 was performed using the Mutector® assay (TrimGen, Sparks, MD, USA). The Mutector® assay is designed for the detection of any type of known DNA mutation.18 In brief, a detection primer is designed that does not permit primer extension when the target base is wild type. As a result, primer extension does not occur, labeled nucleotides are not incorporated, and a color reaction is not observed. When the target base is mutated (eg T → A transversion at BRAF T1796), primer extension continues and a strong color reaction is observed. We used as a template 10-μl from the PCR product of the 102 bp fragment of BRAF exon 15. The assay was preformed according to the manufacturer's instructions. When we previously compared BRAF detection for a large number of human tumors, we found a 100% correlation between the Mutector® assay and direct sequencing.10
As a positive control for the BRAF T1796A mutation, we tested the cutaneous melanoma cell line HTB 72. The cervical cancer cell line ME180, known to be wild type for BRAF at T1796, served as a negative control.
Statistical Analyses
The major statistical end point in this study was the probability of BRAF mutations in papillary thyroid carcinoma across age groups. The strength of the association of BRAF mutation with age category was evaluated with cross tabulations and logistic regression modeling. Cross tabulations were analyzed using the χ2-test. All statistical computations were performed using the SAS system,19 and all P-values reported are two-sided.
Results
In all, 24 patients 18 years or younger with papillary thyroid carcinoma were identified. On review of the histopathologic findings, four of the papillary thyroid carcinomas were classified as follicular variant of papillary thyroid carcinoma, and these were excluded from further analysis. The absence of any prior radiation exposure, either as a form of medical treatment or as a result of living in proximity to Chernobyl during the nuclear accident, was documented by a review of the medical records. One patient had a parent with a history of a sporadic adult papillary thyroid carcinoma, but there was no family history of thyroid malignancy in any of the other 19 patients. The 20 patients with conventional papillary thyroid carcinoma ranged in age from 10 to 17 years (median, 15 years). Totally, 65 adult patients (>18–<40, n=21; ≥40–60, n=29; ≥60, n=15) with conventional papillary thyroid carcinomas were previously evaluated for BRAF mutations using the same Mutector® assay. BRAF mutations were detected in four of the 20 papillary thyroid carcinomas from young patients (Figure 1) compared to 50 of 65 papillary thyroid carcinomas from adult patients (20 vs 77%; OR=13.3, 95% confidence interval (CI)=3.4–56.5; P<0.0001). The frequency of BRAF mutations did not plateau in adult papillary thyroid carcinoma, but continued to rise with advancing patient age (OR as a function of age at 10-year intervals=1.80 CI=1.33–2.44; P<0.001) (Figure 2). For the oldest group of patients with conventional papillary thyroid carcinoma (ie over 60 years of age), the rate of BRAF mutations reached 88%.
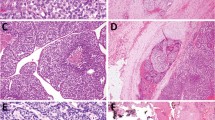
BRAF mutation analysis of papillary thyroid carcinomas. Three examples of adult papillary thyroid carcinomas (PTCs) are shown in the top row (1–3). All of the papillary thyroid carcinomas from young patients are shown in the lower three rows (1–20). For case 3, analysis of both the primary papillary thyroid carcinoma (3P) and its metastatic implant (3M) are shown. Using the Mutector® assay, the presence of a BRAF mutation is indicated by a colorimetric change from clear to green (PTC 2, PTC 3, 3P, 3 M, 10, 11 and 19). In the absence of a mutation, a color signal is not visible. The controls (top row) include the melanoma cell line A2058 (T → A transversion at BRAF T1796) (C+, positive control), the ME 180 cervical cell line (wild-type for BRAF) (C-, negative control), and a water sample without a DNA template (NTC, no template control).
BRAF mutations in sporadic papillary thyroid carcinomas as a function of age. BRAF mutations are much less frequent in papillary carcinomas arising in young patients (less than 20 years of age) than in those carcinomas arising in older patients (20 vs 77%; OR=13.3, 95% confidence interval (CI)=3.4–56.5; P<0.0001).
Discussion
The reported rate of BRAF mutations in papillary thyroid carcinoma is variable, ranging from 29 to 69%.9, 10, 11, 12, 13, 14, 15, 16, 20, 21, 22 This variability is due in large part to the heterogeneous nature of tumors encompassed under the designation of papillary thyroid carcinoma, and likely reflects differences in: (1) phenotypic subtypes of papillary thyroid carcinoma, (2) exposure profiles, and (3) other yet undefined population-based characteristics such as patient age. We found, for example, that when the study sample is narrowed to sporadic conventional papillary thyroid carcinomas from adult patients over the age of 60, the prevalence of BRAF mutations approaches 90%.
The frequency of BRAF mutations is not uniform across all phenotypic variants of papillary thyroid carcinoma. Specifically, it is not frequently detected in the follicular variant of papillary thyroid carcinoma. We had previously noted that only 12% of the follicular variant of papillary thyroid carcinoma harbors BRAF mutations,10 and Trovisco et al15 were unable to identify any BRAF mutations in the 32 follicular variants of papillary thyroid carcinomas that they tested. The reasons underlying these genetic differences are not understood, but some have suggested that the diagnosis of follicular variant of papillary thyroid carcinoma may in fact embrace a spectrum of follicular neoplasms ranging from true carcinomas to low-grade neoplasms with little or no potential for malignant behavior.23, 24, 25 Whatever the case may be, reported prevalence rates are clearly influenced by the composition of the study tumors, especially regarding the proportion of the follicular variant of papillary thyroid carcinoma.
Exposure to radiation also appears to influence prevalence rate of BRAF mutations.21 Whereas BRAF mutation is the predominant mechanism of mitogen-activated protein kinase pathway activation in sporadic papillary thyroid carcinomas, this pathway is preferentially driven by RET/PTC rearrangements in radiation-induced papillary thyroid carcinomas.6, 21, 26 As noncooperative and mutually exclusive events, translocational activation of RET occurs inversely to mutational activation of BRAF in these tumors. In their genetic analysis of mitogen-activated protein kinase activation in radiation-induced papillary thyroid carcinomas, Nikiforova et al observed a high prevalence (58%) of RET/PTC rearrangements, and an inversely low prevalence (4%) of BRAF point mutations.
When dealing with post-Chernobyl papillary thyroid carcinoma, one must consider the potential influence of patient age before attributing a specific genetic profile as a sole reflection of radiation exposure. The differential activation of RET over BRAF in young patients who developed papillary thyroid carcinoma following the Chernobyl nuclear accident is largely attributed to the propensity of radiation to induce double-strand breaks, but RET/PTC rearrangements are disproportionately high in childhood papillary thyroid carcinoma irrespective of any prior radiation exposure.6, 7, 8 Although the specific types of RET/PTC rearrangements may differ, RET activation is clearly the dominant mechanism of mitogen-activated protein kinase pathway activation in both sporadic and radiation-induced childhood papillary thyroid carcinomas.6, 26 Conversely, we found that mutational activation of BRAF plays a less consequential role in sporadic childhood papillary thyroid carcinoma. BRAF mutations were detected in 77% of papillary thyroid carcinomas from adult patients but in only 20% of papillary thyroid carcinomas from patients 18 years or younger. Although the prevalence of BRAF mutations rose dramatically by the 3rd and 4th decades, they continued to gradually increase with each successive decade of life.
The observation that the genetic profile of the most common form of thyroid cancer shifts as a function of age is neither unanticipated nor extraneous. Differences in the behavior of childhood and adult forms of papillary thyroid carcinoma have long been recognized. Compared to their adult counterparts, children with papillary thyroid carcinoma are more likely to develop metastatic spread to regional lymph nodes and to experience, paradoxically, a more favorable clinical outcome.4 Indeed, patient age is a prognostic indicator that is of over-riding importance for patients with papillary thyroid carcinoma. While children with papillary thyroid carcinoma have a life expectancy that parallels their peers without papillary thyroid carcinoma, adult forms of papillary thyroid carcinoma are more likely to be associated with fatal outcomes with the probability of cancer-related mortality increasing exponentially with each decade after the age of 40.1, 5 A better understanding of the genetic alterations underlying thyroid tumorigenesis may help explain these differences in clinical behavior. Whether BRAF mutations directly contribute to the more aggressive nature of adult papillary thyroid carcinoma remains to be established, but Namba et al12 have recently identified BRAF mutations as a marker of advanced tumor stage.
Resolving the patterns of aberrant signal activation along the mitogen-activated protein kinase pathway may also be helpful in individualizing treatment for specific groups of patients with papillary thyroid carcinoma. Targeting of tyrosine kinases is rapidly emerging as a novel strategy for treating various human cancers, and several molecule inhibitors that specifically inactivate individual receptor tyrosine kinases—including inhibitors of key constituents in the MAP kinase pathway—are progressing through clinical trials.27, 28 Our observations suggest that selection of optimal anticancer agents may benefit by taking into account patient age, and, if possible, genetic status of such genes as BRAF. Patients with papillary thyroid carcinoma may not respond in a uniform way to specific inhibitors of the mitogen-activated protein kinase pathway, particularly at the extremes of patient age.
References
Rosai J, Carcangiu ML, DeLellis RA . Papillary carcinoma. In: Rosai J, Carcangiu ML, DeLellis RA (eds). Tumors of the Thyroid Gland 3rd edn. Armed Forces Institute of Pathology: Washington, DC, 1992, pp 65–121.
LiVolsi VA . Unusual variants of papillary thyroid carcinoma. Adv Endocrinol Metab 1995;6:39–54.
Carcangiu ML, Zampi G, Rosai J . Papillary thyroid carcinoma: a study of its many morphologic expressions and clinical correlates. Pathol Annu 1985;20 (Part 1): 1–44.
Zimmerman D, Hay ID, Gough IR, et al. Papillary thyroid carcinoma in children and adults: long-term follow-up of 1039 patients conservatively treated at one institution during three decades. Surgery 1988;104:1157–1166.
Crile Jr G, Hazard JB . Relationship of the age of the patient to the natural history and prognosis of carcinoma of the thyroid. Ann Surg 1953;138:33–38.
Nikiforov YE, Rowland JM, Bove KE, et al. Distinct pattern of ret oncogene rearrangements in morphological variants of radiation-induced and sporadic thyroid papillary carcinomas in children. Cancer Res 1997;57:1690–1694.
Williams GH, Rooney S, Thomas GA, et al. RET activation in adult and childhood papillary thyroid carcinoma using a reverse transcriptase-n-polymerase chain reaction approach on archival-nested material. Br J Cancer 1996;74:585–589.
Jarzab B, Wloch J, Wiench M . Molecular changes in thyroid neoplasia. Folia Histochem Cytobiol 2001;39 (Suppl 2): 26–27.
Cohen Y, Xing M, Mambo E, et al. BRAF mutation in papillary thyroid carcinoma. J Natl Cancer Inst 2003;95:625–627.
Cohen Y, Rosenbaum E, Clark DP, et al. Mutational analysis of BRAF in fine needle aspiration biopsies of the thyroid: a potential application for the preoperative assessment of thyroid nodules. Clin Cancer Res 2004;64:2898–2903.
Kimura ET, Nikiforova MN, Zhu Z, et al. High prevalence of BRAF mutations in thyroid cancer: genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res 2003;63:1454–1457.
Namba H, Nakashima M, Hayashi T, et al. Clinical implication of hot spot BRAF mutation, V599E, in papillary thyroid cancers. J Clin Endocrinol Metab 2003;88:4393–4397.
Soares P, Trovisco V, Rocha AS, et al. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of papillary thyroid carcinoma. Oncogene 2003;22:4578–4580.
Nikiforova MN, Kimura ET, Gandhi M, et al. BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab 2003;88:5399–5404.
Trovisco V, Vieira I DC, Soares P, et al. BRAF mutations are associated with some histological types of papillary thyroid carcinoma. J Pathol 2004;202:247–251.
Puxeddu E, Moretti S, Elisei R, et al. BRAF(V599E) mutation is the leading genetic event in adult sporadic papillary thyroid carcinomas. J Clin Endocrinol Metab 2004;89:2414–2420.
Fearon ER, Feinberg AP, Hamilton SH, et al. Loss of genes on the short arm of chromosome 11 in bladder cancer. Nature 1985;318:377–380.
TrimGen, Genetic Technology [Online] June, 2004. <http://www.trimgen.com/mutector.htmmutector>mutector.
SAS Institute Inc. SAS User's Guide: Statistics 5th edn. SAS Institute, Inc.: Carey, NC, 2004.
Xu X, Quiros RM, Gattuso P, et al. High prevalence of BRAF gene mutation in papillary thyroid carcinomas and thyroid tumor cell lines. Cancer Res 2003;63:4561–4567.
Nikiforova MN, Ciampi R, Salvatore G, et al. Low prevalence of BRAF mutations in radiation-induced thyroid tumors in contrast to sporadic papillary carcinomas. Cancer Lett 2004;209:1–6.
Xing M, Vasko V, Tallini G, et al. BRAF T1796A transversion mutation in various thyroid neoplasms. J Clin Endocrinol Metab 2004;89:1365–1368.
Williams ED, Abrosimov A, Bogdanova T, et al. Guest editorials: two proposals regarding the terminology of thyroid tumors. Int J Surg Pathol 2000;8:181–183.
Renshaw AA, Gould EW . Why there is the tendency to ‘overdiagnose’ the follicular variant of papillary thyroid carcinoma. Am J Clin Pathol 2002;117:19–21.
Chan JK . Strict criteria should be applied in the diagnosis of encapsulated follicular variant of papillary thyroid carcinoma. Am J Clin Pathol 2002;117:16–18.
Rabes HM, Klugbauer S . Molecular genetics of childhood papillary thyroid carcinomas after irradiation: high prevalence of RET rearrangement. Recent Results Cancer Res 1998;154:248–264.
Mercer KE, Pritchard CA . Raf proteins and cancer: B-Raf is identified as a mutational target. Biochim Biophys Acta 2003;1653:25–40.
Tuveson DA, Weber BL, Herlyn M . BRAF as a potential therapeutic target in melanoma and other malignancies. Cancer Cell 2003;4:95–98.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rosenbaum, E., Hosler, G., Zahurak, M. et al. Mutational activation of BRAF is not a major event in sporadic childhood papillary thyroid carcinoma. Mod Pathol 18, 898–902 (2005). https://doi.org/10.1038/modpathol.3800252
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800252
Keywords
This article is cited by
-
Frequent BRAF V600E and Absence of TERT Promoter Mutations Characterize Sporadic Pediatric Papillary Thyroid Carcinomas in Japan
Endocrine Pathology (2017)
-
Classic Architecture with Multicentricity and Local Recurrence, and Absence of TERT Promoter Mutations are Correlates of BRAF V600E Harboring Pediatric Papillary Thyroid Carcinomas
Endocrine Pathology (2016)
-
BRAFV600E mutation is highly prevalent in thyroid carcinomas in the young population in Fukushima: a different oncogenic profile from Chernobyl
Scientific Reports (2015)
-
Mechanisms of Disease: molecular genetics of childhood thyroid cancers
Nature Clinical Practice Endocrinology & Metabolism (2007)
-
Genome-wide gene expression profiling suggests distinct radiation susceptibilities in sporadic and post-Chernobyl papillary thyroid cancers
British Journal of Cancer (2007)