Abstract
Accurate morphologic distinction between small cell carcinoma and poorly differentiated squamous cell carcinoma has critical therapeutic significance, but can be limited by crush artifact, tumor necrosis, limited tumor representation, and overlapping morphologic features. We evaluated a panel of antibodies for their efficacy in distinguishing between these neoplasms. Formalin-fixed paraffin-embedded tissue sections of small cell carcinomas and poorly differentiated squamous cell carcinomas underwent immunohistochemical staining with antibodies to thyroid transcription factor-1, p63, high molecular weight keratin, and p16(INK4A). Of 28 small cell carcinomas, 26 (93%) small cell carcinomas showed diffuse moderate or strong staining for thyroid transcription factor-1 with no staining for high molecular weight keratin and p63. In contrast, 27/28 (96%) poorly differentiated squamous cell carcinomas manifested opposite immunoreactivities, with diffuse moderate or strong staining for high molecular weight keratin and p63, and no or minimal staining for thyroid transcription factor-1. In two additional cases originally interpreted as small cell carcinoma, high molecular weight keratin highlighted small numbers of neoplastic large cells, leading to reclassification as combined small cell and non-small cell carcinomas. p16(INK4A) expression varied widely in poorly differentiated squamous cell carcinomas, but was consistently moderate or strong and diffuse in small cell carcinomas, and proved helpful in the two thyroid transcription factor-1-negative small cell carcinomas. This study demonstrates that a panel consisting of antibodies to thyroid transcription factor-1, p63, high molecular weight keratin, and p16(INK4A) is highly effective for distinguishing between small cell carcinoma and poorly differentiated squamous cell carcinoma. This panel also facilitates diagnosis of combined small cell and non-small cell carcinomas.
Similar content being viewed by others
Main
Current therapeutic strategies for lung cancers require accurate morphologic differentiation between primary pulmonary small cell carcinomas and primary pulmonary non-small cell carcinomas. While morphologic evaluation, supported by immunohistochemical features of epithelial and/or neuroendocrine differentiation, usually is sufficient to accurately classify these cancers, problematic cases are common. Crush artifact, tumor necrosis, and limited tumor representation can preclude definitive morphologic diagnosis. Furthermore, problems in differential diagnosis frequently complicate the morphologic assessment of the combined small cell carcinoma with non-small cell carcinoma variant of small cell carcinoma, and the uncommon small cell squamous cell variant of poorly differentiated squamous cell carcinoma.1
Many investigators have applied immunohistochemical techniques to the evaluation of small cell carcinomas and non-small cell carcinomas. Antibodies directed towards neuroendocrine differentiation, such as chromogranin A, synaptophysin, CD56, and CD57, stain 10–20% of non-small cell carcinomas, and in some reports substantially higher numbers of non-small cell carcinomas, but can be negative in up to 25% of small cell carcinomas.2, 3, 4, 5, 6 Recent studies suggest the potential utility of several newer antibodies including thyroid transcription factor-1 (TTF-1), p63, p16(INK4A) (p16), and high molecular weight keratin, to help differentiate between small cell carcinomas and non-small cell carcinomas.7, 8, 9, 10 In this study, we evaluated the utility of a panel of these antibodies for distinguishing between small cell carcinoma and poorly differentiated squamous cell carcinoma.
Materials and methods
Samples
A total of 60 archival, routinely processed formalin-fixed and paraffin-embedded tissue samples were retrieved from three hospitals affiliated with the University of Texas Health Science Center at Houston Medical School or Baylor College of Medicine, Houston, TX. These samples included 30 small cell carcinomas and 30 poorly differentiated squamous cell carcinomas, obtained as bronchoscopic biopsies (13 cases) or surgical specimens (47 cases).
Histologic Evaluation and Additional Histochemical Staining
All hematoxylin–eosin-stained slides were reviewed by the authors to determine the histologic type of the neoplasm, according to recognized criteria.5 In addition, two cases received mucicarmine staining.
Immunohistochemical Staining
Unstained recuts of 4 μm thickness were obtained from a representative paraffin block from each specimen. Primary antibodies selected for staining included TTF-1 (clone 8G7G3/1, Dako Corp., Carpinteria, CA, USA) optimized at a dilution of 1:60, p63 (clone 7JUL, Novocastra Laboratories Ltd, Newcastle upon Tyne, UK) optimized at a dilution of 1:100, p16 (clone G175-405, B-D Pharmingen, San Diego, CA, USA) optimized at a dilution of 1:7000, and high molecular weight keratin (clone 34βE12, Dako Corp.) optimized at a dilution of 1:20. For TTF-1, p63 and p16, heat-induced epitope retrieval using a steamer (Handy Steamer Plus. Black & Decker, Shelton, CT, USA) was used. The slides were placed in 1 mM EDTA buffer (pH 8.0), steamed for 20 min, and then allowed to cool at room temperature in buffer for 20 min. The Dako Autostainer (Dako Corp.) was used for the remaining steps. The slides were incubated with the primary antibody at room temperature for 30 min. The PowerVision™ (Immuno Vision Technologies Co., Daly City, VA, USA) detection system was used with diaminobenzidine as the chromogen. For high molecular weight keratin, the staining procedure included pretreatment with proteinase K (Dako Corp.) at room temperature for 5 min before incubation with the antibody. The LSAB2 detection system, with diaminobenzidine as the chromogen, was used.
Two cases received additional immunohistochemical staining for surfactant precursor protein B. Heat-induced epitope retrieval was first performed as described earlier, and then the slides were incubated with a 1:15 dilution of surfactant precursor protein B antibody (clone 19H7, Novocastra Laboratories Ltd) at room temperature for 30 min. The LSAB2 detection system (Dako Corp.) was used, and diaminobenzidine was applied as the chromogen.
Identically prepared slides from known positive tissues were used as positive controls for all staining runs. Negative controls were performed for all cases and consisted of identically prepared slides that were treated with antibody diluent (Dako Corp.) in place of primary antibody, but otherwise subjected to the same immunohistochemical staining protocol.
Assessment of Immunohistochemical Staining
Both staining intensity and extensiveness (percentage of tumor cells staining) were evaluated for each antibody applied to each neoplasm. Staining intensity was graded as weak—1+/moderate—2+/strong—3+. Staining extensiveness was scored as 1+ if the percentage of tumor cells stained was less than 10%, 2+ if 10–50% of tumor cells stained, and 3+ if more than 50% of tumor cells stained. Overall reactivity was defined as negative if <10% of tumor cells stained, regardless of staining intensity, low if 1+ staining was observed in 10–50% of tumor cells, intermediate if 2+ or 3+ staining occurred in 10–50% of tumor cells or 1+ staining occurred in more than 50% of tumor cells, and high if 2+ or 3+ staining was present in more than 50% of tumor cells.
Results
Three of the antibodies tested demonstrated markedly different patterns of reactivities for the two types of neoplasms evaluated (Table 1 ). Of 28 small cell carcinomas, 26 (93%) showed diffuse moderate or strong staining for TTF-1 with no staining for high molecular weight keratin and p63 (Figure 1). In 21/26 of the TTF-1-positive small cell carcinomas, more than 90% of tumor cells expressed TTF-1, and the staining intensity was usually strong. In the few TTF-1-positive small cell carcinomas with less than 90% staining, this was usually related to substantial crush artifact or less optimal fixation in the centers of resected neoplasms. In contrast, 27/28 (96%) poorly differentiated squamous cell carcinomas manifested the opposite pattern of reactivities, with diffuse moderate or strong staining for high molecular weight keratin and p63, and no or minimal staining for TTF-1 (Figure 2). While high molecular weight keratin staining increased with increased squamoid differentiation, p63 staining was inversely related to keratinization, making p63 particularly useful as a marker for squamous differentiation in the most poorly differentiated squamous cell carcinomas. Seven of the poorly differentiated squamous cell carcinomas included a component of small cell variant of squamous cell carcinoma, which showed the same pattern of immunohistochemical staining as the conventional poorly differentiated squamous cell carcinomas.
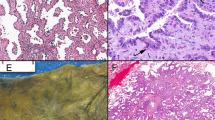
Small cell carcinomas usually showed moderate or strong staining for p16 by more than 90% of the neoplastic cells (27/28 cases; Table 1), while loss of p16 expression was common in poorly differentiated squamous cell carcinomas. Although p16 expression overlapped between the two groups of neoplasms, p16 staining was helpful in one particular circumstance: in the two TTF-1-negative small cell carcinomas, which were also nonreactive for p63 and high molecular weight keratin, p16 was strongly and diffusely expressed (Figure 3). Thus, for TTF-1-negative small cell carcinomas, p16 staining may be useful for supporting a diagnosis of small cell carcinoma.
The four cases eliminated from the original cohort of 60 highlighted two additional benefits of using the panel of antibodies studied. In two cases originally interpreted as small cell carcinoma, over 90% of tumor cells displayed strong (3+) and diffuse (3+) nuclear staining for TTF-1 and p16. Interestingly, high molecular weight keratin highlighted small numbers of neoplastic large cells (<10% of total cells in each case) that showed moderate (2+) p63 reactivity in one case and no p63 reactivity in the other case (Figure 4). These observations led to the reclassification of these two cases as combined small cell carcinoma/non-small cell carcinoma. In two other cases originally diagnosed as poorly differentiated squamous cell carcinoma, tumor cells demonstrated diffuse moderate or strong TTF-1 staining, no reactivity with high molecular weight keratin and p63, and diffuse weak staining for p16, a pattern of reactivities more consistent with the diagnosis of small cell carcinoma than poorly differentiated squamous cell carcinoma (Figure 5). However, by light microscopy, both tumors were poorly differentiated large cell neoplasms with a solid pattern. On subsequent staining, both cases expressed surfactant precursor protein B and in one, neoplastic cells contained intracytoplasmic mucin. The mucin-positive case was reclassified as a poorly differentiated adenocarcinoma, and the mucin-negative case was reclassified as a large cell carcinoma, with immunohistochemical evidence of type II pneumocyte/Clara cell differentiation. In both of these cases, discrepancy between the morphologic impression and the staining results led to further evaluation and reclassification.
Poorly differentiated adenocarcinoma. Histology (a), immunohistochemical stains for TTF-1 (b), p63 (c), high molecular weight keratin (d), and p16 (e), mucicarmine (f and inset), and immunohistochemical stain for surfactant precursor protein B (g) (all except inset, original magnification × 200; inset, original magnification × 400).
Discussion
For patients with lung cancer, pathologic classification of the neoplasm as small cell carcinoma or non-small cell carcinoma remains a key decision point in the therapeutic algorithm. Distinction between small cell carcinomas and poorly differentiated squamous cell carcinomas, however, can be difficult due to a variety of endogenous and procedural factors. Particular challenges may occur in diagnosis of the combined small cell carcinoma with non-small cell carcinoma variant of small cell carcinoma, and distinction between the small cell squamous cell variant of poorly differentiated squamous cell carcinoma and small cell carcinoma. Although chromatin characteristics can help to direct one to the correct diagnosis, chromatin detail is often impaired by crush artifact, necrosis and less than optimal preservation. Other cytologic characteristics such as cell and nuclear size, and amount of cytoplasm, may overlap. Furthermore, presence or absence of neuroendocrine differentiation, as determined using immunohistochemical or ultrastructural methods, does not separate these groups of neoplasms.
Recently, however, reports have appeared suggesting the utility of several newer antibodies for distinguishing between small cell carcinomas and poorly differentiated squamous cell carcinomas. From among this group, we selected the particular antibodies for the current panel with the intent of providing both positive and negative stains for both entities, as well as the capacity to draw attention to neoplasms in which a third diagnosis should be considered. A similar approach has been used by Lantuejoul et al11 to distinguish between basaloid and large cell neuroendocrine carcinomas of the lung.
Most small cell carcinomas display a TTF-1+/p16+/p63−/ high molecular weight keratin− immunophenotype, while most poorly differentiated squamous cell carcinomas show opposite reactivities for three of the antibodies (TTF-1−/p63+/ high molecular weight keratin+) and variable retention or loss of p16 expression. Tumor components of small cell variant of squamous cell carcinoma showed the same staining properties as conventional poorly differentiated squamous cell carcinomas. Our results confirm and extend the recent work of Wu and colleagues, who reported negative or rarely equivocal immunohistochemical reactivity for p63 in 23/23 small cell carcinomas compared with diffuse intense staining for p63 in 13/13 poorly differentiated squamous cell carcinomas, as well as TTF-1 staining in 20/23 small cell carcinomas and 0/13 poorly differentiated squamous cell carcinomas.8 Others have also reported high frequencies (generally >85% of cases) of TTF-1 expression by pulmonary small cell carcinomas as well as many pulmonary adenocarcinomas.9, 12, 13, 14, 15, 16, 17, 18, 19 Interestingly, in two of our original cohort of cases, observation of TTF-1 staining, in combination with a lack of p63 and high molecular weight keratin staining, led to reconsideration of the diagnosis, additional mucicarmine and surfactant precursor protein B staining, and reclassification. TTF-1 is a transcription factor that is normally expressed in alveolar pneumocytes, Clara cells, ciliated respiratory epithelial cells, and basal cells of the lung,9 and is also expressed in the thyroid and brain.20 p63 is a p53 homologue mapped to chromosome 3q27, that is commonly amplified early in the development of pulmonary squamous cell carcinoma.21 In the non-neoplastic lung, p63 is normally expressed by bronchial reserve cells, and lower strata of metaplastic squamous epithelia, and it may play a role in stem cell commitment in squamous epithelium.22
The high molecular weight keratin antibody recognizes cytokeratins 1, 5, 10, and 14 in the Moll catalog23 and reacts with airway basal cells and metaplastic squamous cells. Morphologic small cell carcinomas showing the typical immunohistochemical profile (TTF-1+/p16+/p63−), except for reactivity of some cells with high molecular weight keratin, should be carefully scrutinized for populations of large neoplastic cells. Our results support the recent report by Sturm et al,10 who noted the utility of this stain for highlighting small populations of neoplastic large cells in predominant small cell carcinomas that were not obviously combined neoplasms histologically.
Lastly, p16 contributed least to the efficacy of the panel, but was helpful for supporting a diagnosis of small cell carcinoma in two neoplasms demonstrating the expected nonreactivity with p63 and high molecular weight keratin, and no reactivity with TTF-1. Over the last 10 years, increasing data have emerged supporting the importance of p16 in the development of neoplasms of the lungs and numerous other sites. The protein is an inhibitor of the cyclin-dependent kinase that catalyzes the phosphorylation of the retinoblastoma gene protein. Disruption of the retinoblastoma pathway of cell cycle arrest appears to occur in most non-small cell carcinomas, through p16 inactivation and/or upregulation of cyclin D1.24, 25, 26 Aberrant methylation of p16 appears to represent an early event in pulmonary carcinogenesis and is associated with p16 inactivation and loss of p16 expression.27, 28 In contrast to pulmonary small cell carcinomas, which largely retain p16 expression, pulmonary squamous cell carcinomas have demonstrated more frequent loss of expression of p16.29, 30, 31
In summary, a primary immunohistochemistry panel including TTF-1, p63, and high molecular weight keratin is an efficient and powerful supplement to morphology for distinguishing between small cell carcinoma and poorly differentiated squamous cell carcinoma. This panel revealed two distinctively different patterns of reactivities for small cell carcinomas and poorly differentiated squamous cell carcinomas, including small cell variant neoplasms. In cases deviating from the usual reactivity patterns, second tier evaluation of p16 or mucin and surfactant precursor protein B (depending upon the morphology) offers additional support for diagnoses of small cell carcinoma or adenocarcinoma/large cell carcinoma, respectively. High molecular weight keratin is also useful for highlighting minor populations of large cells in combined small cell carcinomas with non-small cell carcinomas that are comprised predominantly of small neoplastic cells.
References
Cagle PT . Tumors of the lung (excluding lymphoid tumors). In: Thurlbeck WM, Churg AM (eds). Pathology of the Lung, 2nd edn. Thieme Medical Publishers: New York, 1995, pp 437–551.
Abbona G, Papotti M, Viberti L, et al. Chromogranin A gene expression in non-small cell lung carcinomas. J Pathol 1998;186:151–156.
Baldi A, Groger AM, Esposito V, et al. Neuroendocrine differentiation in non-small cell lung carcinomas. In Vivo 2000;14:109–114.
Guinee Jr DG, Fishback NF, Koss MN, et al. The spectrum of immunohistochemical staining of small-cell lung carcinoma in specimens from transbronchial and open-lung biopsies. Am J Clin Pathol 1994;102:406–414.
Travis WD, Colby TV, Corrin B . Histological Typing of Lung and Pleural Tumours, 3rd edn. Springer-Verlag: Berlin, 1999.
Loy TS, Darkow GV, Quesenberry JT . Immunostaining in the diagnosis of pulmonary neuroendocrine carcinomas. An immunohistochemical study with ultrastructural correlations. Am J Surg Pathol 1995;19:173–182.
Lyda MH, Weiss LM . Immunoreactivity for epithelial and neuroendocrine antibodies are useful in the differential diagnosis of lung carcinomas. Hum Pathol 2000;31:980–987.
Wu M, Wang B, Gil J, et al. p63 and TTF-1 immunostaining. A useful marker panel for distinguishing small cell carcinoma of lung from poorly differentiated squamous cell carcinoma of lung. Am J Clin Pathol 2003;119:696–702.
Nakamura N, Miyagi E, Murata S, et al. Expression of thyroid transcription factor-1 in normal and neoplastic lung tissues. Mod Pathol 2002;15:1058–1067.
Sturm N, Rossi G, Lantuejoul S, et al. 34BetaE12 expression along the whole spectrum of neuroendocrine proliferations of the lung, from neuroendocrine cell hyperplasia to small cell carcinoma. Histopathology 2003;42:156–166.
Lantuejoul S, Sturm N, Laverriere MH, et al. Thyroid transcription factor-1 and cytokeratin 1, 5, 10, 14 (34BetaE12) expression in basaloid and large cell neuroendocrine carcinomas of the lung. Mod Pathol 2001;14:222A.
Byrd-Gloster AL, Khoor A, Glass LF, et al. Differential expression of thyroid transcription factor 1 in small cell lung carcinoma and Merkel cell tumor. Hum Pathol 2000;31:58–62.
Cheuk W, Kwan MY, Suster S, et al. Immunostaining for thyroid transcription factor 1 and cytokeratin 20 aids the distinction of small cell carcinoma from Merkel cell carcinoma, but not pulmonary from extrapulmonary small cell carcinomas. Arch Pathol Lab Med 2001;125:228–231.
Folpe AL, Gown AM, Lamps LW, et al. Thyroid transcription factor-1: immunohistochemical evaluation in pulmonary neuroendocrine tumors. Mod Pathol 1999;12:5–8.
Ordonez NG . Value of thyroid transcription factor-1 immunostaining in distinguishing small cell lung carcinomas from other small cell carcinomas. Am J Surg Pathol 2000;24:1217–1223.
Sturm N, Rossi G, Lantuejoul S, et al. Expression of thyroid transcription factor-1 in the spectrum of neuroendocrine cell lung proliferations with special interest in carcinoids. Hum Pathol 2002;33:175–182.
Zamecnik J, Kodet R . Value of thyroid transcription factor-1 and surfactant apoprotein A in the differential diagnosis of pulmonary carcinomas: a study of 109 cases. Virchows Arch 2002;440:353–361.
Pelosi G, Fraggetta F, Pasini F, et al. Immunoreactivity for thyroid transcription factor-1 in stage I non-small cell carcinomas of the lung. Am J Surg Pathol 2001;25:363–372.
Tan D, Li Q, Deeb G, et al. Thyroid transcription factor-1 expression prevalence and its clinical implications in non-small cell lung cancer: a high-throughput tissue microarray and immunohistochemistry study. Hum Pathol 2003;34:597–604.
Bingle CD . Thyroid transcription factor-1. Int J Biochem Cell Biol 1997;29:1471–1473.
Massion PP, Taflan PM, Jamshedur Rahman SM, et al. Significance of p63 amplification and overexpression in lung cancer development and prognosis. Cancer Res 2003;63:7113–7121.
Wang BY, Gil J, Kaufman D, et al. P63 in pulmonary epithelium, pulmonary squamous neoplasms, and other pulmonary tumors. Hum Pathol 2002;33:921–926.
Moll R, Franke WW, Schiller DL, et al. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell 1982;31:11–24.
Brambilla E, Gazzeri S, Moro D, et al. Alterations of Rb pathway (Rb-p16INK4-cyclin D1) in preinvasive bronchial lesions. Clin Cancer Res 1999;5:243–250.
Brambilla E, Moro D, Gazzeri S, et al. Alterations of expression of Rb, p16(INK4A) and cyclin D1 in non-small cell lung carcinoma and their clinical significance. J Pathol 1999;188:351–360.
Nuovo GJ, Plaia TW, Belinsky SA, et al. In situ detection of the hypermethylation-induced inactivation of the p16 gene as an early event in oncogenesis. Proc Natl Acad Sci USA 1999;96:12754–12759.
Belinsky SA, Nikula KJ, Palmisano WA, et al. Aberrant methylation of p16(INK4a) is an early event in lung cancer and a potential biomarker for early diagnosis. Proc Natl Acad Sci USA 1998;95:11891–11896.
Zochbauer-Muller S, Fong KM, Virmani AK, et al. Aberrant promoter methylation of multiple genes in non-small cell lung cancers. Cancer Res 2001;61:249–255.
Beasley MB, Lantuejoul S, Abbondanzo S, et al. The P16/cyclin D1/Rb pathway in neuroendocrine tumors of the lung. Hum Pathol 2003;34:136–142.
Huang CI, Taki T, Higashiyama M, et al. p16 protein expression is associated with a poor prognosis in squamous cell carcinoma of the lung. Br J Cancer 2000;82:374–380.
Leversha MA, Fielding P, Watson S, et al. Expression of p53, pRB, and p16 in lung tumours: a validation study on tissue microarrays. J Pathol 2003;200:610–619.
Acknowledgements
We thank Bonnie Price Whitaker, H.T. (ASCP) for her expert technical assistance with immunohistochemistry.
Author information
Authors and Affiliations
Corresponding author
Additional information
Duality of interest
The authors have no duality of interest to declare.
Rights and permissions
About this article
Cite this article
Zhang, H., Liu, J., Cagle, P. et al. Distinction of pulmonary small cell carcinoma from poorly differentiated squamous cell carcinoma: an immunohistochemical approach. Mod Pathol 18, 111–118 (2005). https://doi.org/10.1038/modpathol.3800251
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/modpathol.3800251
Keywords
This article is cited by
-
Integrative analysis of mRNA-miRNA-TFs reveals the key regulatory connections involved in basal cell carcinoma
Archives of Dermatological Research (2020)
-
Human papillomavirus-related small cell carcinoma of the oropharynx: a case report and literature review
Cancers of the Head & Neck (2017)
-
p16INK4a overexpression is associated with CDKN2A mutation and worse prognosis in HPV-negative laryngeal squamous cell carcinomas
Virchows Archiv (2015)