Abstract
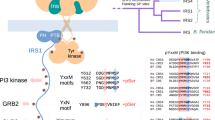
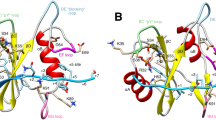
THE domain organization of many signalling proteins facilitates a segregation of binding, catalytic and regulatory functions1,2. The mammalian SH2 domain protein tyrosine phosphatases (PTPs) contain tandem SH2 domains and a single carboxy-terminal catalytic domain3. SH-PTP1 (PTP1C, HCP) and SH-PTP2 (Syp, PTP2C, PTP1D) function downstream from tyrosine kinase-Iinked insulin, growth factor, cytokine and antigen receptors4–12. As well as directing subcellular localization by binding to receptors and their substrates, the two SH2 domains of these PTPs function together to regulate catalysis7,13,14. Here we report the structure of the tandem SH2 domains of SH-PTP2 in complex with monophosphopeptides. A fixed relative orientation of the two domains, stabilized by a disulphide bond and a small hydrophobic patch within the interface, separates the peptide binding sites by ~ 40 Å. The defined orientation of the SH2 domains in the structure, and data showing that peptide orientation and spacing between binding sites is critical for enzymatic activation, suggest that spatial constraints are important in this multidomain protein–protein interaction.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Pawson, T. Nature 373, 573–580 (1995).
Cohen, G. B., Ren, R. & Baltimore, D. Cell 80, 237–248 (1995).
Feng, G.-S. & Pawson, T. Trends Genet. 10, 54–58 (1993).
Kuhne, M. R., Pawson, T., Lienhard, G. E. & Feng, G.-S. J. biol. Chem. 288, 11479–11481 (1993).
Feng, G.-S., Hui, C.-C. & Pawson, T. Science 259, 1607–1614 (1993).
Vogel, W., Lammers, R., Huang, J. & Ullrich, A. Science 259, 1611–1614 (1993).
Lechleider, R. J. et al. J. biol. Chem. 268, 21478–21481 (1993).
Kazlauskas, A., Feng, G. S., Pawson, T. & Valius, M. Proc. natn. Acad. Sci. U.S.A. 90, 6939–6942 (1993).
Yi, T. L., Mui, A. L.-F., Krystal, G. & Ihle, J. N. Molec. cell. Biol. 13, 7577–7586 (1993).
Klingmuller, U., Lorenz, U., Cantley, L. C., Neel, B. G. & Lodish, H. F. Cell 80, 729–738 (1995).
D'Ambrosio, D. et al. Science 268, 293–296 (1995).
Doody, G. M. et al. Science 269, 242–244 (1995).
Sugimoto, S., Wandless, T., Shoelson, S. E., Neel, B. G. & Walsh, C. T. J. biol. Chem. 269, 13614–13622 (1994).
Pluskey, S., Wandless, T. J., Walsh, C. T. & Shoelson, S. E. J. biol. Chem. 270, 2897–2900 (1995).
Lee, C.-H. et al. Structure 2, 423–438 (1994).
Lechleider, R. J., Freeman, R. M. & Neel, B. G. J. biol. Chem. 268, 13434–13438 (1993).
Hatada, M. H. et al. Nature 377, 32–38 (1995).
Shiue, L., Zoller, M. J. & Brugge, J. S. J. biol. Chem. 270, 10498–10502 (1995).
Backer, J. M. et al. EMBO J. 11, 3469–3479 (1992).
Carpenter, C. L. et al. J. biol. Chem. 268, 9478–9483 (1993).
Hu, Q., Klippel, A., Muslin, A. J., Fantl, W. J. & Williams, L. T. Science 268, 100–102 (1995).
Case, R. D. et al. J. biol. Chem. 269, 10467–10474 (1994).
Kabsch, W. J. J. appl. Crystallogr. 21, 916–924 (1988).
Navaza, J. in Molecular Replacement: Proceedings of the CCP4 Study Weekend (ed. Dodson, E. J., Gover, S. & Wolf, W.) 87–90 (SERC, Daresbury Laboratory, Warrington, 1992).
Brunger, A. T. X-PLOR Version 3.1: A System for X-ray Crystallography and NMR (Yale Univ. Press, New Haven, CT, 1992).
Jones, T. A., Zou, J. Y., Cowan, S. W., & Kjeldgaard, M. Acta Crystallogr. 47, 110–119 (1991).
Lamzin, V. S. & Wilson, K. S. Acta crystallogr. D49, 129–147 (1993).
Eck, M. J., Shoelson, S. E. & Harrison, S. C. Nature 362, 87–91 (1993).
Kraulis, P. J. Appl. Crystallogr. 24, 946–950 (1991).
Nicholls, A., Sharp, K. A. & Honig, B. Proteins Struct. Funct. Genet. 11, 281–296 (1995).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Eck, M., Pluskey, S., Trüb, T. et al. Spatial constraints on the recognition of phosphoproteins by the tandem SH2 domains of the phosphatase SH-PTP2. Nature 379, 277–280 (1996). https://doi.org/10.1038/379277a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/379277a0
This article is cited by
-
The tyrosine phosphatase SHP2 increases robustness and information transfer within IL-6-induced JAK/STAT signalling
Cell Communication and Signaling (2021)
-
Grb2 binding induces phosphorylation-independent activation of Shp2
Communications Biology (2021)
-
Interaction of SHP-2 SH2 domains with PD-1 ITSM induces PD-1 dimerization and SHP-2 activation
Communications Biology (2020)
-
Inhibition of the Gab2/PI3K/mTOR signaling ameliorates myeloid malignancy caused by Ptpn11 (Shp2) gain-of-function mutations
Leukemia (2017)
-
Structural insights into Noonan/LEOPARD syndrome-related mutants of protein-tyrosine phosphatase SHP2 (PTPN11)
BMC Structural Biology (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.