Abstract
The retinoic acid receptor (RAR) β gene is a putative tumor suppressor gene on chromosome 3p24, where a high incidence of loss of heterozygosity is detected in many types of tumors. Retinoic acid suppresses cancer cell growth through binding to RARs, especially RARβ, indicating a critical role in mediating anticancer effects. Selective loss or down-regulation of RARβ mRNA and protein has been reported in prostate cancers (PCas), although the mechanisms remain unclear. We investigated the role of epigenetic modification in RARβ2 gene silencing. Aberrant methylation was detected in 11 of 14 (79%) primary PCas, 9 of 10 (90%) hormone-refractory PCas, and 2 of 4 (50%) PCa cell lines, but not in any normal prostate samples. Chromatin immunoprecipitation assay showed that all RARβ2-negative cells (LNCaP, PC3, and DU145) were hypoacetylated at both histones H3 and H4. After exposure to 5-aza-2prime;-deoxycytidine treatment, Trichostatin A and all-trans retinoic acid induced partial demethylation, increased accumulation of acetylated histones, and markedly restored the expression of RARβ2 in RARβ2-negative cells. These data suggest that the RARβ2 gene may be one of the frequently silenced genes by epigenetic modifications in PCa.
Similar content being viewed by others
Introduction
Retinoids are inhibitors of tumorigenesis, with effects mediated by binding to nuclear retinoid receptors. Nuclear retinoid receptors comprise two different families: the retinoic acid receptors (RARs) and the retinoid X receptors (RXRs), with three subtypes for each (α, β, and γ) (Chambon, 1996). Each subtype has several isoforms resulting from different promoter usage and alternative splicing. Among these receptors, RARβ, or more specifically, the isoform β2, is decreased or down-regulated in a number of tumors, including lung, breast, and esophageal cancers, and squamous cell carcinomas of the head and neck (Lotan et al, 1995; Picard et al, 1999; Qiu et al, 1999; Xu et al, 1997). Additionally, RARβ mRNA and protein are selectively lost in prostate cancer (PCa) tissues (Lotan et al, 2000). The level of RARβ transcripts increases in many cell types in response to all-trans retinoic acid (ATRA). This is caused by the presence of several retinoic acid responsive elements (RAREs) within the RARβ promoter region, where the nuclear hormone receptor heterodimer RAR/RXR binds (de The et al, 1990). Exogenous expression of the RARβ gene in RARβ-negative cancer cells increases their responsiveness to growth inhibition and induction of apoptosis by retinoic acid (RA) (Sun et al, 2000). However, little is known about the mechanisms underlying the silencing of RARβ expression in tumor cells.
The RARβ gene is characterized by two different promoters and transcripts, which are produced by alternative splicing. Most human cells express RARβ2 as the major isoform. The RARβ2 promoter is characterized by a CpG (cytidine phosphate guanosine)-rich region, the CpG island (Gardiner-Garden and Frommer, 1987), which is located in the 5′-untranslated region, along with several motifs that are potential binding sites for transcription factors such as AP-1, AP-2, and Sp1. Additionally, RAREs, βRAREs, and a TATA box are located near the transcription initiation site (Baust et al, 1996; van der Leede et al, 1992).
DNA methylation, especially in the CpG-rich promoter regions, inhibits transcription by interfering with initiation or by reducing the binding affinity of sequence-specific transcription factors (reviewed in Bird and Wolffe, 1999). Recently, it was demonstrated that methyl-CpG binding proteins recruit transcription repressors such as histone deacetylase (Jones et al, 1998; Nan et al, 1998; Ng et al, 1999; Wade et al, 1999).
To clarify the epigenetic mechanism of RARβ2 gene regulation in PCas, we detailed the methylation status of the RARβ2 promoter region using the bisulfite PCR method and histone acetylation status associated with promoter region by chromatin immunoprecipitation (ChIP) assay.
Results
Methylation Analysis of the RARβ2 Promoter Region
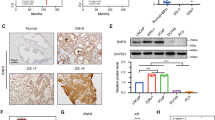
Using bisulfite PCR direct sequencing and methylation-specific PCR (MSP) methods, approximately 1 kb of the RARβ2 promoter region (Fig. 1) (−477/+392, GenBank accession numbers S82362 and M96016) containing 38 CpGs was examined for its methylation status. In four PCa cell lines, LNCaP cells were densely methylated in the entire region around the CpG island. PC3 cells were heterogeneously methylated in regions 2 and 3, but not in region 1 (Fig. 2, A and B). The most commonly methylated CpG sites were numbers 21, 22, and 23 in region 2 (Fig. 2B). We constructed specific MSP primers to detect the methylation status of these CpG sites. In DU145, TSU-Pr1, and all normal prostate samples, aberrant methylation was not detected by bisulfite direct sequencing in these regions (Fig. 2B). MSP analysis indicated hypermethylation in LNCaP and PC3, and no methylation in normal prostate samples, DU145, or TSU-Pr1 (Fig. 2C). These results were consistent with bisulfite sequencing data.
5′-untranslated region of the retinoic acid receptor (RAR) β2 gene. Top, The cytidine phosphate guanosine (CpG) island covers approximately 1 kb of the RARβ2 gene 5′-untranslated region, containing 38 CpGs (numbered 1 to 38). Several transcription factor binding sites are also indicated. Bottom, Sequence of β retinoic acid responsive element (RARE) region including three CpG dinucleotides.
A, Bisulfite sequencing. Partial sequencing data for LNCaP and PC3 cells are presented. Direct sequencing data revealed that single “C” peaks at the corresponding CpG sites were complete methylations, single “T” peaks were nonmethylations, and overlapping “C” and “T” peaks were partial or heterogenous methylations. B, Summarized bisulfite sequencing of the RARβ2 promoter region in prostate cancer (PCa) cell lines and tissues. At least 10 clones from each cell line and tissue were analyzed. C, Methylation-specific PCR (MSP) analysis. MSP analysis was performed using specific primers for bisulfite-modified DNA. Reactions specific for methylated DNA (M) or for unmethylated DNA (U) are indicated.
Using a laser-capture microdissection (LCM) system, three foci were microdissected in each clinical sample and DNA was extracted from each focus. When methylation was detected in more than one focus by MSP, the clinical specimen was classified as methylation-positive (Fig. 3). In tumor specimens, 11 of 14 primary PCas (79%) and 9 of 10 refractory PCas (90%) had hypermethylation of the RARβ2 promoter region. All samples were confirmed by bisulfite sequencing (Fig. 2B). The relationships between methylation and clinicopathologic factors are summarized in Table1.
Laser-capture microdissection (LCM). A, Human PCa specimens before microdissection. B, Human PCa specimens after microdissection. C, Microdissection of cancerous focus. D, In PCa samples, three foci were microdissected and analyzed by MSP. Reactions specific for methylated DNA (M) or for unmethylated DNA (U) are indicated.
Expression of RARβ2 in Normal Prostate Samples, PCa Samples, and PCa Cell Lines
Only one of four PCa cell lines (TSU-Prl) expressed RARβ2 mRNA, the other three cell lines (LNCaP, PC3, and DU145) were essentially negative for RARβ2 mRNA (Fig. 4A). In normal prostate tissues, RARβ2 was expressed at various levels. In primary PCas, loss or down-regulation of RARβ2 expression was observed in five of eight cases (Fig. 4B). Regarding the methylation status of the RARβ2 promoter, positive PCa samples (PC5, 7, and 8) were not methylated. All normal prostate samples expressed RARβ2 and were not methylated.
RARβ2 expression. A, RT-PCR analysis of RARβ2 expression in normal prostate tissue and four PCa cell lines. B, RT-PCR analysis of RARβ2 expression in eight PCa samples. GAPDH was used as an internal control. NP, normal prostate tissue; L, LNCaP cells; P, PC3 cells; D, DU145 cells; T, TSU-Pr1 cells; NC, negative control.
RARβ2 Re-expression with Demethylating Agent and Histone Deacetylase Inhibitor Treatment
To clarify the role of epigenetic suppression of the RARβ2 gene, we treated PCa cell lines with 5-aza-2′-deoxycitidine (azaC), Trichostatin A (TSA), and ATRA. With single agents, RARβ2 re-expression was not detected in RARβ2-negative cells (data not shown). However, combined treatment with azaC and ATRA induced slight RARβ2 gene re-expression in LNCaP and PC3 cells, and after azaC treatment, the combination of TSA and ATRA caused a marked increase in RARβ2 gene re-expression. In DU145 cells, re-expression of RARβ2 was not detected with azaC and ATRA treatments, but was observed with addition of TSA and ATRA after azaC treatment (Fig. 5A). To determine the effects of the demethylating agent, we examined the methylation status in LNCaP and PC3 cells after azaC treatment. In both cell lines, partial demethylation was detected by MSP (Fig. 5B).
RARβ2 re-expression. A, RARβ2 re-expression by the treatment of chromatin remodeling drugs. The treatment of 5-aza-2′-deoxycitidine (azaC), Trichostatin A (TSA), and all-trans retinoic acid (ATRA) is described in “Materials and Methods”. B, Demethylation after combined drug treatment. MSP analysis showed partial demethylation of the RARβ2 promoter region after azaC and ATRA treatment. These experiments were performed three times, each with similar results.
ChIP Assay
Using a ChIP assay, we examined the local histone acetylation in the chromatin associated with the RARβ2 core promoter region (−165/+27), which includes TATA and RAREs sequences and three of the common methylated CpGs of the RARβ2-negative LNCaP and PC3 cells. Figure 6 shows the results of the acetyl-histone H3 and H4 immunoprecipitation assay with or without drug treatment. In TSU-Pr1, RARβ2-positive cells, 192 bp of unmethylated RARβ2 promoter region was amplified after immunoprecipitation. However, in all RARβ2-negative cells, this region failed to be amplified, but after the combination of azaC, TSA, and ATRA treatments, accumulation of acetylated histones was found. Regardless of the drug treatment, the 166 bp of the GAPDH promoter region was amplified after ChIP.
Chromatin immunoprecipitation (ChIP) assay. ChIP, with the use of antibodies to acetylated histone H3 and H4, detected both histones acetylations of the RARβ2 promoter in the RARβ2-positive TSU-Pr1 cell line and of the GAPDH promoter in all cell lines. After the combined treatment with TSA and ATRA after azaC exposure, histone H3 and H4 acetylation was increased in all RARβ2-negative cell lines. These experiments were performed three times, each with similar results.
Discussion
The results of the present study demonstrated that the 5′ CpG island in the RARβ2 gene promoter region was methylated in two RARβ2-negative PCa cell lines and in more than 80% of human PCa samples. Hypermethylation of the RARβ2 gene has been reported in breast and lung cancers (Sirchia et al, 2000; Virmani et al, 2000; Widschwendter et al, 2000). Arapshian et al (2000) concluded that methylation of the RARE region may be particularly important in RARβ2 gene silencing. Here, we show for the first time that the RARβ2 gene is one of the genes involved in aberrant methylation in human PCas. Additionally, the methylated promoter region of the RARβ2-negative PCa cell lines (LNCaP and PC3) was associated with hypoacetylation of both histones H3 and H4.
DNA methylation is an important mechanism in PCas, and is involved in the inactivation of various essential genes such as E-cadherin, glutathione S-transferase P1, the endothelin B receptor, and p16/CDKN2 (Graff et al, 1995; Jarrard et al, 1997; Lee et al, 1994; Nelson et al, 1997). In the RARβ2 gene, the percentages of samples that showed hypermethylation were 79% (11 of 14) in primary PCas and 90%(9 of 10) in hormone-refractory PCas, but 0%(0 of 10) in normal prostate samples. In our samples, no correlations were found between methylation status and clinicopathologic factors. We conclude that hypermethylation of the RARβ2 gene leading to loss of RARβ2 expression may be an early event during malignant progression.
In the present study, we used a LCM system for avoiding contamination with normal cells, such as lymphocytes and stromal cells. Figure 3 shows heterogeneous methylation in a clinical sample. We speculate that these heterogenous CpG methylation patterns were mainly caused by heterogenous cell populations of tumor cells.
Bisulfite sequencing data indicated that three CpG sites (numbers 20 to 22) near the βRARE region are consensus regions of methylation in PCas. We speculate that methylation of these CpGs may be critical for the silencing of the gene by blocking access of liganded RAR/RXR heterodimers and other cis-acting transcription factors to their binding sequences. In two of methylated, RARβ2-negative PCa cell lines (LNCaP and PC3), azaC and ATRA induced RARβ2 re-expression and partial demethylation. Hypomethylated DU145 cells were not restored. However, in combination with TSA, RARβ2 expression was markedly increased in all negative cells.
The mechanisms of epigenetic change, especially the chromatin structural changes during the silencing of the genes, is not fully understood. Acetylation and deacetylation on lysine residues of histone amino-terminal tails have profound effects on gene transcription (reviewed in Strahl and Allis, 2000). Transcriptional repression is induced by deacetylation of the core histones H3 and H4 (reviewed in Wolffe et al, 2000). Thus, we examined histone acetylation associated with RARβ2 promoter region. ChIP assay detected a loss of acetylated histones H3 and H4 in all RARβ2-negative cells. A recent study demonstrated that synergistic effects of local histone deacetylation and DNA hypermethylation are crucial factors for chromatin structure alteration leading to transcriptional suppression (Jones et al, 1998; Nan et al, 1998; Ng et al, 1999; Wade et al, 1999). Methylated DNA can be silenced by methyl-CpG binding proteins, such as MeCP2-recruiting Sin3A/histone deacetylase complex. Cameron et al (1999) demonstrated that histone hypoacetylation occurs in the aberrantly methylated promoter. In our previous experiments, we showed that the androgen receptor gene was hypermethylated in an androgen receptor-negative PCa cell line (DU145) and that re-activation was induced by combined azaC and TSA treatments (Nakayama et al, 2000). In the chromatin of the hypermethylated androgen receptor minimal promoter region, both histones H3 and H4 were hypoacetylated (T. Nakayama, M. Watanabe, unpublished data). The RARβ2 gene may be also silenced by methylation-dependent epigenetic mechanisms in some RARβ2-negative cells. In our experiments, expression of RARβ2 was restored in hypomethylated DU145 cells by inhibition of histone deacetylases but not by demethylation, suggesting that a silencing mechanism such as methylation-independent histone deacetylation may be important in this case. Such inactivations were previously reported for p21/Waf1 gene inactivation in human cancer cells (Richon et al, 2000; Shin et al, 2000).
We demonstrated that there was a loss of acetylation of histones H3 and H4 associated with RARβ2 promoter methylation, and that combined TSA and ATRA treatment after azaC treatment increased the accumulation of acetylated histones leading to re-activation of methylated RARβ2 promoter. These data indicate that promoter hypermethylation may be secondary to the transcriptional repression and may lead to a stable inactive chromatin state, similar to the inactive X chromosome, the imprinted gene locus, and BRCA1 gene inactivation (Jeppesen and Turner, 1993; Rice and Futscher, 2000; Saitoh and Wada, 2000). Further study is necessary to clarify the precise mechanisms whereby DNA hypermethylation and histone deacetylation are involved in the alteration of chromatin structure and promoter inactivation.
In conclusion, we demonstrated aberrant methylation of the RARβ2 gene in a majority of clinical PCa samples and in PCa cell lines. Such methylation seems to be specific to cancer because it was not detected in normal prostate samples, and therefore, could potentially serve as a good molecular marker for early cancer detection. We also demonstrated that RARβ2 might be silenced not only by DNA methylation but also by histone deacetylation. Combined treatment with azaC, ATRA, and TSA may be an effective therapeutic strategy to treat PCa by restoring RARβ2 mRNA expression.
Materials and Methods
Cell Culture and Drug Treatment
PCa cell lines (LNCaP, PC3, and DU145) were obtained from the American Type Tissue Culture Collection (Rockville, Maryland). TSU-Pr1 was kindly provided by Dr. W. B. Isaacs (Johns Hopkins University School of Medicine, Baltimore, Maryland). All cell lines were cultured routinely in RPMI1640 with 10% FBS or 10% charcoal-dextran–stripped FBS at 37° C with 5% CO2. AzaC (Sigma Chemical Company, St. Louis, Missouri) treatment was performed at 1 μm for 72 hours, TSA (100 ng/ml; Sigma) and ATRA (1 μm; Sigma) treatments were for 24 hours. For the combination of azaC, TSA, and ATRA treatments, azaC was introduced for an initial incubation of 48 hours, and ATRA or TSA/ATRA were added for an additional 24 hours.
Tissue Samples
Ten samples of normal prostate tissue were obtained at autopsy. All samples were examined by histopathology and determined to have no evidence of cancerous lesions. Fourteen primary PCa specimens were obtained at radical prostatectomy at Mie University Hospital, Mie, Japan, snap-frozen and stored at −80° C. Additionally, ten hormone-refractory tumors were obtained from distant organ site metastases at autopsy at Chiba University Hospital, Chiba, Japan, and genomic DNA was immediately extracted by a standard protocol. All ten of the hormone-refractory tumor patients had experienced a new onset of cancer under hormonal therapy and died. For RNA extraction, we selected eight of the primary PCa samples and microdissected parts of the specimens composed of more than 80% tumor cells. The clinicopathologic findings for the examined samples are summarized in Table 1.
Tissue Preparation and Sampling by LCM
Frozen tissues were cut into 4 to 6 μm-thick sections with a cryostat. Sections were placed onto glass slides that had been baked at 230° C for 4 hours. The sections were immediately fixed with 70% ethanol for 10 minutes and then washed with diethyl pyrocarbonate-treated water for 5 seconds. Sections were stained with hematoxylin for 15 seconds, washed with diethyl pyrocarbonate-treated water for 10 seconds, dehydrated with an ethanol gradient, and counterstained with an alcoholic Eosin Y solution for 30 seconds. Sections were washed three times with 100% ethanol and three times with xylenes. The sections were air dried with a fan for 20 minutes and stored in a plastic container with silica gel at −80° C until use.
Frozen tumor/or adjacent normal tissues were microdissected using a Pixcell LCM system (LM200; Arcturus Engineering Inc., Mountain View, California). Sections were covered with LCM transfer film (Capture TF-100; Arcturus Engineering Inc.), and specific portions of the histologic section were affixed to the capture film by brief laser pulses. DNA and RNA were extracted as described previously (Goldsworthy et al, 1999; Hayes et al, 2000).
Bisulfite Modification
Genomic DNA (approximately 0.5 μg) was treated with sodium bisulfite as described previously (Frommer et al, 1992). After denaturation in 0.3 M NaOH at 37° C for 15 minutes, sodium bisulfite was added to a final concentration of 3.1 M, and hydroquinone was added to a final concentration of 0.5 mm. The reaction was performed at 55° C for 16 hours, and desalted using the Wizard DNA purification resin (Promega, Madison, Wisconsin) according to the manufacturer's instructions. Bisulfite modification was completed by 0.3 M NaOH treatment at 37° C for 15 minutes. Modified DNA was precipitated with ethanol, washed in 70% ethanol, dried, and resuspended in 50 μl of distilled water.
Bisulfite Sequencing and Methylation-Specific PCR (MSP)
The methylation status of the 5′-regulatory region of RARβ2 in PCa cell lines was analyzed by bisulfite PCR sequencing and MSP as described previously (Frommer et al, 1992; Herman et al, 1996). Bisulfite genomic sequencing was performed with the following primers: Region 1 (product size, 355 bp): forward, 5′-GTA TGT GTT TTT TTT GGA GTG G-3′, reverse, 5′-AAC TTA AAA ACT CCC AAC AAC C-3′; Region 2 (product size, 154 bp): forward, 5′-TGG GAG TTG GTG ATG TTA GA-3′, reverse, 5′-ACC CTC CTA ACC TCT AAA CA-3′; Region 3 (product size, 391 bp): forward, 5′-TGT TTA GAG GTT AGG GTT TAT T-3′, reverse, 5′-AAC TCC ATC AAA CTC TAC CCC TT-3′. No CpG dinucleotide motifs were contained in these primer sequences. PCR conditions were as follows: 95° C for 10 minutes, 30 cycles of 95° C for 30 seconds, 57° C for 30 seconds, 72° C for 30 seconds, and 72° C for 10 minutes. For bisulfite genomic sequencing, total PCR products were gel-purified and directly sequenced using the ABI 310 automated sequencing system (Perkin Elmer, Foster City, California).
For MSP analysis, modified DNA was amplified with specific-primers: 5′-GGG TTT ATC GAA AGT TTA TTC-3′ (forward-methylated) and 5′-TTC CGA ATA CGT TCC GAA T-3′ (reverse-methylated); 5′-GGT AGG GTT TAT TGA AAG TTT ATT T-3′ (forward-unmethylated) and 5′-AAA CCT TCC AAA TAC ATT CCA AAT-3′ (reverse-unmethylated). PCR was carried out under the following conditions: 95° C for 10 minutes then 30 amplification cycles (95° C for 30 seconds, 59° C for 30 seconds, 72° C for 30 seconds) and a final extension incubation of 10 minutes at 72° C. PCR products were directly loaded on 2.0% agarose gels and analyzed after ethidium-bromide staining.
RT-PCR for RARβ2
Total RNA was prepared using Isogen (Nippon Gene, Tokyo, Japan), according to the manufacturer's instructions. Aliquots of 2 μg of total RNA were used for generation of cDNAs using Superscript reverse transcriptase (GIBCO BRL, Gaithersburg, Maryland). The specific primers applied to detect RARβ2 transcripts (GenBank accession number X07282) were as follows: forward (located in exon 3), 5′-GCA TGG CAG AGT GCC CTA TC-3′; reverse (located in exon 6), 5′-TCC CAG AGT CAT CCC TGC TTC AT-3′. PCR amplification was performed for 30 cycles at 95° C for 30 seconds, 62° C for 30 seconds, and 72° C for 60 seconds. Human GAPDH was used as an internal control. The PCR products were subjected to electrophoresis in 2.0% agarose gels and visualized by ethidium bromide staining.
ChIP Assay
ChIP assays using antibodies to acetyl-histone H3 and H4 were performed according to the manufacturer's instructions (Upstate Biotechnology, Lake Placid, New York). Cells were cultured and treated with 100 ng/ml of TSA and/or 1 μm ATRA for 24 hours after 1 μm azaC treatment for 48 hours. Formaldehyde was then added to the cells to a final concentration of 1% and incubated at 37° C for 10 minutes. The cells were washed in 1 ml of ice-cold PBS with proteinase inhibitors, scraped, resuspended in 200 μl of SDS lysis buffer, and incubated on ice for 10 minutes. Lysates were sonicated for 10 seconds three times on ice and centrifuged at 15,000 rpm for 10 minutes at 4° C. Supernatants were loaded on 1% agarose gels and determined to have reduced DNA lengths to between 200 and 1000 bp. Sonicated samples were diluted 10-fold with immunoprecipitation buffer and divided equally to prepare negative control (no antibody) immunoprecipitation samples. The samples were precleaned with a salmon sperm DNA/protein A agarose slurry and incubated overnight at 4° C with or without antibodies to histone H3 or H4. Chromatin-antibody complexes were collected using a salmon sperm DNA/protein A agarose slurry and washed according to the manufacturer's protocol. Immunocomplexes were eluted twice with 250 μl of elution buffer (1% SDS, 0.1 M NaHCO3) for 15 minutes at room temperature. To reverse crosslinks, 20 μl of 5 M NaCl were added with incubation for 4 hours at 65° C. Ten microliters of 0.5 M EDTA, 20 μl of 1 M Tris-HCl pH 6.5, and 2 μl of 10 mg/ml Proteinase K were added, and the samples were incubated at 45° C for 1 hour. Immunoprecipitated DNA was recovered by phenol/chloroform extraction and ethanol precipitation and analyzed by PCR. The primer pairs used for ChIP analysis of the RARβ2 promoter region (GenBank accession numbers S82362 and M96016, PCR product length 192 bp) were 5′-CTC TGG CTG TCT GCT TTT GC-3′ (forward), 5′-CAG CTC ACT TCC TAC TAC TTC-3′ (reverse). The primers used for the GAPDH promoter region (GenBank accession number J04038, PCR product length 166 bp) were 5′-TAC TAG CGG TTT TAC GGG CG-3′ (forward), 5′-TCG AAC AGG AGG AGC AGA GA-3′ (reverse). PCR was performed for 25 to 30 cycles of 95° C for 30 seconds, 58° C for 30 seconds, and 72° C for 30 seconds. PCR products were analyzed on 2.0% agarose gels and visualized by UV illumination.
References
Arapshian A, Kuppumbatti YS, and Mira-y-Lopez R (2000). Methylation of conserved CpG sites neighboring the beta retinoic acid response element may mediate retinoic acid receptor beta gene silencing in MCF-7 breast cancer cells. Oncogene 19: 4066–4070.
Baust C, Redpath L, and Schwarz E (1996). Different ligand responsiveness of human retinoic-acid-receptor beta-gene transcription in tumorigenic and non-tumorigenic cervical-carcinoma-derived cell lines is mediated through a large retinoic-acid-response domain. Int J Cancer 67: 409–416.
Bird AP and Wolffe AP (1999). Methylation-induced repression: Belts, braces, and chromatin. Cell 99: 451–454.
Cameron EE, Bachman KE, Myöhänen S, Herman JG, and Baylin SB (1999). Synergy of demethylation and histone deacetylase inhibition in the re-expression of genes silenced in cancer. Nat Genet 21: 103–107.
Chambon P (1996). A decade of molecular biology of retinoic acid receptors. FASEB J 10: 940–954.
de The H, Vivanco-Ruiz MM, Tiollais P, Stunnenberg H, and Dejean A (1990). Identification of a retinoic acid responsive element in the retinoic acid receptor beta gene. Nature 343: 177–180.
Frommer M, McDonald LE, Millar DS, Collis CM, Watt F, Grigg GW, Molloy PL, and Paul CL (1992). A genomic sequencing protocol that yields a positive display of 5-methylcytosine residues in individual DNA strands. Proc Natl Acad Sci USA 89: 1827–1831.
Gardiner-Garden M and Frommer M (1987). CpG islands in vertebrate genomes. J Mol Biol 196: 261–282.
Graff JR, Herman JG, Lapidus RG, Chopra H, Xu R, Jarrard DF, Isaacs WB, Pitha PM, Davidson NE, and Baylin SB (1995) E-cadherin expression is silenced by DNA hypermethylation in human breast and prostate carcinomas. Cancer Res 55: 5195–5199.
Goldsworthy SM, Stockton PS, Trempus CS, Foley JF, and Maronpot RR (1999). Effects of fixation on RNA extraction and amplification from laser capture microdissected tissue. Mol Carcinog 25: 86–91.
Hayes AJ, Huang W-Q, Yu J, Maisonpierre PC, Liu A, Kern FG, Lippman ME, McLeskey SW, and Li L-Y (2000) Expression and function of angiopoietin-1 in breast cancer. Br J Cancer 83: 1154–1160.
Herman JG, Graff JR, Myohanen S, Nelkin BD, and Baylin SB (1996). Methylation-specific PCR: A novel PCR assay for methylation status of CpG islands. Proc Natl Acad Sci USA 93: 9821–9826.
Jarrard DF, Bova GS, Ewing CM, Pin SS, Nguyen SH, Baylin SB, Cairns P, Sidransky D, Herman JG, and Isaacs WB (1997). Deletional, mutational, and methylation analyses of CDKN2 (p16/MTS1) in primary and metastatic prostate cancer. Genes Chromosomes Cancer 19: 90–96.
Jeppesen P and Turner BM (1993). The inactive X chromosome in female mammals is distinguished by a lack of histone H4 acetylation, a cytogenetic marker for gene expression. Cell 74: 281–289
Jones PL, Veenstra GJ, Wade PA, Vermaak D, Kass SU, Landsberger N, Strouboulis J, and Wolffe AP (1998). Methylated DNA and MeCP2 recruit histone deacetylase to repress transcription. Nat Genet 19: 187–191.
Lee WH, Morton RA, Epstein JI, Brooks JD, Campbell PA, Bova GS, Hsieh WS, Isaacs WB, and Nelson WG (1994). Cytidine methylation of regulatory sequences near the pi-class glutathione S-transferase gene accompanies human prostatic carcinogenesis. Proc Natl Acad Sci USA 91: 11733–11737.
Lotan R, Xu XC, Lippman SM, Ro JY, Lee JS, Lee JJ, and Hong WK (1995). Suppression of retinoic acid receptor-beta in premalignant oral lesions and its up-regulation by isotretinoin. N Engl J Med 332: 1405–1410.
Lotan Y, Xu XC, Shalev M, Lotan R, Williams R, Wheeler TM, Thompson TC, and Kadmon D (2000). Differential expression of nuclear retinoid receptors in normal and malignant prostates. J Clin Oncol 18: 116–121.
Nan X, Ng HH, Johnson CA, Laherty CD, Turner BM, Eisenman RN, and Bird A (1998). Transcriptional repression by the methyl-CpG-binding protein MeCP2 involves a histone deacetylase complex. Nature 393: 386–389.
Nakayama T, Watanabe M, Suzuki H, Toyota M, Sekita N, Hirokawa Y, Mizokami A, Ito H, Yatani R, and Shiraishi T (2000). Epigenetic regulation of androgen receptor gene expression in human prostate cancers. Lab Invest 80: 1789–1796.
Nelson JB, Lee WH, Nguyen SH, Jarrard DF, Brooks JD, Magnuson SR, Opgenorth TJ, Nelson WG, and Bova GS (1997). Methylation of the 5′ CpG island of the endothelin B receptor gene is common in human prostate cancer. Cancer Res 57: 35–37.
Ng HH, Zhang Y, Hendrich B, Johnson CA, Turner BM, Erdjument-Bromage H, Tempst P, Reinberg D, and Bird A (1999). MBD2 is a transcriptional repressor belonging to the MeCP1 histone deacetylase complex. Nat Genet 23: 58–61.
Picard E, Seguin C, Monhoven N, Rochette-Egly C, Siat J, Borrelly J, Martinet Y, Martinet N, and Vignaud JM (1999). Expression of retinoid receptor genes and proteins in non-small-cell lung cancer. J Natl Cancer Inst 91: 1059–1066.
Qiu H, Zhang W, El-Naggar AK, Lippman SM, Lin P, Lotan R, and Xu XC (1999). Loss of retinoic acid receptor-beta expression is an early event during esophageal carcinogenesis. Am J Pathol 155: 1519–1523.
Rice JC and Futscher BW (2000). Transcriptional repression of BRCA1 by aberrant cytosine methylation, histone hypoacetylation and chromatin condensation of the BRCA1 promoter. Nucleic Acids Res 28: 3233–3239.
Richon VM, Sandhoff TW, Rifkind RA, and Marks PA (2000). Histone deacetylase inhibitor selectively induces p21WAF1 expression and gene-associated histone acetylation. Proc Natl Acad Sci USA 97: 10014–10019.
Saitoh S and Wada T (2000). Parent-of-origin specific histone acetylation and reactivation of a key imprinted gene locus in Prader-Willi syndrome. Am J Hum Genet 66: 1958–1962.
Shin JY, Kim HS, Park J, Park JB, and Lee JY (2000). Mechanism for inactivation of the KIP family cyclin-dependent kinase inhibitor genes in gastric cancer cells. Cancer Res 60: 262–265.
Sirchia SM, Ferguson AT, Sironi E, Subramanyan S, Orlandi R, Sukumar S, and Sacchi N (2000). Evidence of epigenetic changes affecting the chromatin state of the retinoic acid receptor β2 promoter in breast cancer cells. Oncogene 19: 1556–1563.
Strahl BD and Allis CD (2000). The language of covalent histone modifications. Nature 403: 41–45.
Sun SY, Wan H, Yue P, Hong WK, and Lotan R (2000). Evidence that retinoic acid receptor beta induction by retinoids is important for tumor cell growth inhibition. J Biol Chem 275: 17149–17153.
van der Leede BJ, Folkers GE, Kruyt FA, and van der Saag PT (1992). Genomic organization of the human retinoic acid receptor β2. Biochem Biophys Res Commun 188: 695–702.
Virmani AK, Rathi A, Zochbauer-Muller S, Sacchi N, Fukuyama Y, Bryant D, Maitra A, Heda S, Fong KM, Thunnissen F, Minna JD, and Gazdar AF (2000). Promoter methylation and silencing of the retinoic acid receptor-beta gene in lung carcinomas. J Natl Cancer Inst 92: 1303–1307.
Wade PA, Gegonne A, Jones PL, Ballestar E, Aubry F, and Wolffe AP (1999). Mi-2 complex couples DNA methylation to chromatin remodelling and histone. Nat Genet 23: 62–66.
Widschwendter M, Berger J, Hermann M, Muller HM, Amberger A, Zeschnigk M, Widschwendter A, Abendstein B, Zeimet AG, Daxenbichler G, and Marth C (2000). Methylation and silencing of the retinoic acid receptor-β2 gene in breast cancer. J Natl Cancer Inst 92: 826–832.
Wolffe AP, Urnov FD, and Guschin D (2000). Co-repressor complexes and remodelling chromatin for repression. Biochem Soc Trans 28: 379–386.
Xu XC, Sneige N, Liu X, Nandagiri R, Lee JJ, Lukmanji F, Hortobagyi G, Lippman SM, Dhingra K, and Lotan R (1997). Progressive decrease in nuclear retinoic acid receptor beta messenger RNA level during breast carcinogenesis. Cancer Res 57: 4992–4996.
Acknowledgements
We are grateful to Dr. M. Toyota (Department of Molecular Biology, Cancer Research Institute, Sapporo Medical University School of Medicine, Hokkaido, Japan) for constructive comments during the preparation of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakayama, T., Watanabe, M., Yamanaka, M. et al. The Role of Epigenetic Modifications in Retinoic Acid Receptor β2 Gene Expression in Human Prostate Cancers. Lab Invest 81, 1049–1057 (2001). https://doi.org/10.1038/labinvest.3780316
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.3780316
This article is cited by
-
Enhancement of tumor suppressor RAR-β protein expression by cationic liposomal-ATRA treatment in benzo(a)pyrene-induced lung cancer mice model
Naunyn-Schmiedeberg's Archives of Pharmacology (2019)
-
The association between Histone 3 Lysine 27 Trimethylation (H3K27me3) and prostate cancer: relationship with clinicopathological parameters
BMC Cancer (2014)
-
Epigenetic alteration of p16 and retinoic acid receptor beta genes in the development of epithelial ovarian carcinoma
Tumor Biology (2014)