Abstract
Inflammatory myofibroblastic tumor (IMT) is composed of myofibroblasts, plasma cells, and lymphocytes. Cytokines are possibly involved in its pathogenesis. Human herpesvirus-8 (HHV-8) encodes cell cycle regulatory and signaling proteins. A combination of nested PCR with several negative controls and Southern blot methods showed the presence of HHV-8 DNA in seven cases of IMT. Additionally, strong expression was demonstrated by in situ hybridization in many tumoral nuclei. Most of the myofibroblasts in all of the cases were immunoreactive for human IL-6 and cyclin D1. These cytokines probably have a paracrine action and may sustain myofibroblastic growth. HHV-8 could play an essential role in triggering IMT development by a local reactivation of viral lytic replication. The relationship between HHV-8 and immunosuppression status as the only associated cause for tumorigenesis should be revised.
Similar content being viewed by others
Introduction
Inflammatory myofibroblastic tumor (IMT), also known as inflammatory pseudotumor, is a “tumor composed of differentiated myofibroblastic spindle cells usually accompanied by numerous plasma cells and/or lymphocytes” (Weiss, 1994). It was originally described in the lung, but it now seems that extrapulmonary IMT can occur in virtually any anatomic location and at any age. There are many unanswered questions about the pathogenesis of IMT as an exaggerated reactive process or a true neoplasm. The frequent clinical findings of fever, night sweats, fatigue, weight loss, and lymphadenopathy as well as the laboratory findings of polyclonal hypergammaglobulinemia and elevation in erythrocyte sedimentation rate suggest an infectious or autoimmune cause (Arber et al, 1998). However, bacterial, fungal, or viral organisms have only occasionally been detected within IMT (Matsubara et al, 1988).
A recently described gammaherpesvirus, Kaposi's sarcoma (KS) -associated herpesvirus (KSHV) or human herpesvirus-8 (HHV-8) has been detected in 95% of KS from patients with or without AIDS (Chang et al, 1994; Gómez-Román et al, 1999; Marchioli et al, 1996). This herpesvirus is unique in encoding a number of proteins mimicking cell cycle regulatory and signaling proteins such as bcl-2, cyclin-D, interferon regulatory factors, and IL-6 (Cesarman and Knowles, 1997). Such molecules have been involved in the pathogenesis of KS, and IL-6 has a function in the proliferation of AIDS-KS cells, keratinocytes, renal mesangial cells, smooth muscle cells, and myoblasts (Kishimoto et al, 1992). There is growing consensus that HHV-8 is the infectious cofactor required for all forms of KS (Lin et al, 1996), although it is not known whether HHV-8 is a true transforming virus or if it only has an enhancing role in tumorigenesis (Gallo, 1998).
Some reports have suggested that IL-6 may be involved in the pathogenesis of IMT (Coffin et al, 1998b), because of the frequent finding of increased serum IL-6 levels in these patients (Kishimoto et al, 1992). As far as we know, there are no reports about the possible relationship between HHV-8 and IMT. This report describes the presence of HHV-8 DNA sequences and cytokine overexpression in seven cases of IMT.
Results
The seven IMT cases were comprised of five men and two women with ages ranging from 19 to 79 years (average age: 49.6 years). Five cases were located in the lung, one in one limb, and one in the retroperitoneal lymph nodes. Two of the seven patients showed systemic symptoms like fever or leucocytosis. Viral serology was performed preoperatively in only one patient, where no antibodies against Epstein-Barr virus, cytomegalovirus, or varicela zoster virus were detected. We reviewed the clinical charts of all of the cases and none of them showed a record of continuous infections or immunosuppression.
All but one of the tumors were surgically removed. That case (#6) was a 19-year-old male with a large conglomerate mass formed by lymph nodes involving retroperitoneum, both kidneys, and aortic branches. This patient underwent a laparoscopy with biopsy and, after the diagnosis was established, had a good clinical response after treatment with steroids.
The six tumors resected were grossly large whitish masses, with a whorled appearance, and a firm surface on cut section. The borders frequently mingled with adjacent non-neoplastic tissue. By light microscopy, they were composed of myofibroblastic spindle cells immunoreactive for vimentin and α-smooth muscle actin and negative for epithelial markers (cytokeratins and epithelial membrane antigen). In several of these cases, numerous plasma cells and lymphocytes were evident in some tumoral areas. Pleomorphisms, necrosis, or atypical mitotic figures were not found. Clinical follow-up for at least six months revealed no evidence of relapses or deaths.
HHV-8 sequences were detected in paraffin-embedded material by three repeats of nested PCR. Nested PCR was performed in a blinded fashion, with several positive and negative controls. Positive signals were also observed in two AIDS-KS specimens and in two pleural KS lesions without AIDS (positive controls). Amplification was detected neither in non-neoplastic skin samples from healthy individuals nor in the HL-60 cell line (negative controls) (Fig. 1, panel A1). Additionally, peripheral blood mononuclear cells from ten healthy individuals, which were used as negative controls, were negative for HHV-8 (data not shown). Southern blot hybridization confirmed these results (Fig. 1, panel A2). HHV-8 amplification was confirmed using primer sets amplifying non-overlapping regions of the KSHV major capsid gene, as previously suggested (Moore et al, 1996b) (Fig. 1, panels B and C).
Electrophoresis of the PCR products showing the expected 233-bp band from the HHV-8 (panel A1), and the corresponding Southern blot with the internal probe (panel A2). Confirmation with either primer Set 1 (213 bp) (panel B) or Set 2 (115 bp) (panel C), which amplify non-overlapping regions of the HHV-8 hypothetical major capsid gene (see “Materials and Methods”). Note that the C5 lane is not present in panel B. The C5 lane contains DNA from a pleural KS biopsy; we could not obtain sufficient DNA from this sample. Lane M: marker (123-bp leader); lanes C1 and C2: dermal KS lesions (positive controls); lane C3: HL-60 cell line (negative control); lanes 1 to 7: inflammatory myofibroblastic tumors; lanes C4 and C5: pleural KS lesions (positive controls); lanes C6 to C8: skin biopsies from healthy individuals (negative controls).
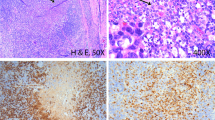
In situ hybridization (ISH) showed a strong HHV-8 expression in many nuclei of spindle-shaped cells in the seven cases examined (Fig. 2A), indicating that the results obtained by PCR and Southern blot were not caused by non-neoplastic cells.
A: In situ hybridization with HHV-8 probe. Strong expression in nuclei of myofibroblasts in IMT (original magnification, × 100). B and C: Immunoreactivity for human IL-6. Intense immunoreactivity in most myofibroblastic cells as well as in endothelial cells and in the lymphocytic population (original magnification, × 100). D: Immunoreactivity for human cyclin D1. Intense nuclear immunoreactivity (original magnification, × 100).
Immunostaining for human IL-6 revealed intense immunoreactivity in most of the spindle myofibroblastic cells as well as in endothelial cells and in the lymphocytic population mingled with spindle cells (Fig. 2, B and C). This pattern was present in all of the seven cases. Human cyclin D1 showed variable nuclear reactivity in most of the myofibroblastic cells in the seven cases (Fig. 2D).
Discussion
IMT is composed of differentiated myofibroblastic spindle cells accompanied by numerous plasma cells and/or lymphocytes (Weiss, 1994). IMT are most frequently found in the lung, but can occur at any age in any anatomic location (Coffin et al, 1998b). The clinical onset may be insidious or rapid, and is accompanied by a constitutional syndrome of fever, weight loss, and laboratory abnormalities in 15% to 30% of cases (Coffin et al, 1998b). Two of our patients (28.6%) showed this constitutional syndrome, which disappeared after surgical resection of the mass and steroid treatment, as is reported in the literature. These clinical and laboratory features suggest that cytokines such as IL-6 may be involved in the pathogenesis of IMT (Coffin et al, 1998b).
The main cellular component in IMT is the spindle myofibroblastic cell. These are ubiquitous cells; whenever tissue injury occurs, indigenous fibroblasts transform to myofibroblasts. This scenario is under the control of several cytokines and growth factors (Coffin et al, 1998a), and IL-6 is one of the most important (Kishimoto et al, 1992). IMT, by itself, seems to be an important source of IL-6. This could explain the high amounts of IL-6 and IL-1β in both tissue and peripheral blood in a child with a plasma cell granuloma of the lung (Rohrlich et al, 1995).
Some reports have identified infectious agents in IMT, including bacterial, rickettsial, fungal, or viral agents, but only in isolated cases (Matsubara et al, 1988). Whether such a purported infection causes the IMT is uncertain. The role of a triggering agent, if any, could be restricted to the early stages of the disease. It might initiate a cascade of paracrine reactions in which stromal cells and plasma cells stimulate each other in a reciprocal exchange, after which the tumor would become self-perpetuating. The production of soluble mediators resulting from this cascade could then induce the systemic manifestations of the disease (Matsubara et al, 1988).
HHV-8 has been detected in 95% of KS tumor specimens from patients with AIDS (Chang et al, 1994; Marchioli et al, 1996) as well as in immunosuppressed patients without AIDS (Gómez-Román et al, 1999). HHV-8 has been also associated with body cavity B-cell lymphoma, benign lymphoproliferative disease, angiosarcoma of the face, angiolymphoid hyperplasia with eosinophilia, multicentric Castleman's disease (Marchioli et al, 1996; Foreman et al, 1997), and sarcoidosis (DiAlberti et al, 1997). There have also been isolated cases of HHV-8 DNA sequences in patients without immunosuppression or KS (Memar et al, 1997; Trovato et al, 1999), although there is a general agreement that HHV-8 pathogenic effects are enhanced by immunosuppression. Interestingly, HHV-8 was found in the interfollicular regions of hyperplastic lymph nodes and in endothelial cells and pneumocytes of the lung in nonimmunosuppressed patients (Trovato et al, 1999). Both locations are implicated in our cases of pulmonary and ganglionar IMT.
Recently, the sequence of the long unique coding region of HHV-8 was reported (Russo et al, 1996). This virus was found to encode homologues to a variety of cellular genes, such as viral cyclin D (Cesarman et al, 1998) and IL-6 (Moore et al, 1996a). This viral IL-6 has been shown to be functional in proliferation assays and is expressed in HHV-8–infected hematopoietic and endothelial cells (Moore et al, 1996a). Viral IL-6 can also mediate signal transduction through the human IL-6 receptor (Wan et al, 1999), and can start a cascade of paracrine reactions involving human IL-6 and other inflammatory cytokines implicated in the inhibition of cellular apoptosis (IL-6) and the transcription of DNA synthesis genes (cyclin D). Although a specific search for viral cyclin D and IL-6 was not directly addressed in this report, clear immunoreactivity for both human cyclin D and IL-6 was demonstrated with murine monoclonal antibodies. Although a polyclonal rabbit antibody raised against viral IL-6 peptides does not cross-react with human IL-6 (Parravicini et al, 1997), it is unknown whether antibodies to human IL-6 cross-react with the homologous viral product.
The exact oncogenic mechanism of HHV-8 is unknown. Most of the cell cycle regulatory genes that are responsible for tumorigenesis in other herpesviruses are latency-associated genes (Ganem, 1997). Whatever the operational mechanism is in the pathogenesis of the HHV-8–associated diseases, it could be explained by viral reactivation in latently infected cells rather than by a primary lytic infection (Cesarman and Knowles, 1997; Gilison and Ambinder, 1997). As with other human herpesviruses, many factors like immunosuppression, viral dose, genetics, viral coinfection, and inflammatory cytokines might interact in the pathogenesis, morphology, and biologic aggressivity of diseases associated with HHV-8 (Chang et al, 2000). The lytic cycle of HHV-8 might directly contribute to initial tumor pathogenesis because most of the HHV-8–encoded homologues of cellular cytokines and anti-apoptotic factors are expressed during the lytic cycle (Sun et al, 1999). Our findings, in a tumor such as IMT, which is characterized by an autolimited growth, viral particles in many tumoral cells, and an overexpression of human cytokines with an important inflammatory background, probably favor a localized reactivation of HHV-8 lytic replication that would undergo a self-perpetuating myofibroblastic neoplasia.
In summary, we report the presence of HHV-8 DNA sequences in myofibroblastic cells in seven cases of pulmonary and ganglionar IMT. This correlates with an overexpression of human IL-6 and human cyclin D1 in the IMT tissues. Human IL-6 originating in IMT may be the factor responsible for systemic symptoms in some cases of IMT. The present report does not show whether HHV-8 behaves as a transforming virus, but it can be hypothesized that HHV-8 plays an essential role in initial IMT development. This first report of HHV-8 associated with a non-lymphoid neoplastic growth in an immunocompetent host indicates that the relationship between HHV-8 and immunosuppression as the only associated cause for tumorigenesis must be revised.
Materials and Methods
Case Reports and Tissue Samples
Seven cases of IMT from three different locations (lung, soft tissue, and lymph nodes) were retrieved from our files. All of them were reviewed in a blinded fashion by two of the authors (EHN and JJGR) to confirm the diagnosis of IMT. In our study, we included only cases that showed the classic morphologic and immunohistochemical picture previously described (Weiss, 1994). The clinical records of the patients were reviewed, personal data of interest checked, and the patients were followed for at least six months postoperative. The clinicopathologic characteristics are presented in Table 1. Tissue samples were obtained from surgical specimens. In all cases, the material obtained was fixed in 10% buffered formalin for 24 hours. Routine procedures were followed for dehydration and subsequent embedding in paraffin. Control negative samples for HHV-8 were obtained from skin and peripheral blood of healthy individuals. Control positive samples for HHV-8 were obtained from skin and pleural KS specimens previously described (Gómez-Román et al, 1999).
DNA Extraction
DNA was extracted by using the NucleoSpin C+T (Macherey-Nagel Gmbh, Düren, Germany). DNA from each specimen was obtained in a laboratory that was physically isolated from the room where the PCR analyses were performed. The PCR amplification was repeated up to three times to assure the reproducibility of the results. Samples were analyzed in a blinded fashion. Stringent laboratory conditions and appropriate controls (see “Results” section) were used to avoid cross-contamination and false-positive results. All samples were tested for amplification using specific primers for exon eight of the C1 inhibitor gene and for the HLA-DQ locus (GH26/GH27).
PCR and Southern Blot Analysis
A nested-PCR detection of HHV-8 was performed as previously described (Moore et al, 1996b). We used a primer set amplifying a 571-bp fragment (sense and antisense, respectively): KS4 (5′-AGCACTCGCA- GGGCAGTACG-3′) and KS5 (5′-GACTCTTCGCT- GATGAACTGG-3′). The amplification of the external region was carried out for 2 minutes at 94° C for initial denaturation, 35 cycles at 94° C for 30 seconds, 60° C for 30 seconds, 72° C for 45 seconds, and 72° C for 5 minutes as final extension. PCR product (1 to 3 μl) was added to an inner PCR reaction mixture and amplified for 25 additional cycles with the use of the primers amplifying the 233-bp KS330233 region of the sequence associated with HHV-8: KS1 (5′-AGCCGAAA- GGATTCCACCAT-3′) and KS2 (5′-TCCGTGTTG- TCTACGTCCAG-3′). Amplifications were performed in a Perkin-Elmer 9600 Thermalcycler (Perkin-Elmer Cetus, Norwalk, Connecticut). Amplification products were analyzed for the presence or absence of the expected 233-bp band on a 2% agarose gel containing ethidium bromide. Confirmation of HHV-8 amplification was performed by using primer sets amplifying non-overlapping regions of the HHV-8 major capsid gene, as suggested (Moore et al, 1996a, 1996b). Primary determination of sample positivity was made with primers amplifying the KS330233 region and was confirmed with two sets of primers directed against non-overlapping regions of the HHV-8 hypothetical major capsid gene with the same PCR conditions as above: Set 1 (outer sense 5′-AGGCAACGTC- AGATGTGAC-3′, outer antisense 5′-GAAATTACC- CACGAGATCGC-3′, inner sense 5′- CATGGG- AGTACATTGTCAGGACCTC-3′, inner antisense 5′-GGAATTATCTCGCAGGTTGCC-3′), which generates a 213-bp PCR product; and Set 2 (outer sense 5′-GGCGACATTCATCAACCTCAGG-3′, outer antisense 5′-ATATCATCCTGTGCGTTCACGAC-3′, inner sense 5′- CGCATGGAGGACCTAGTCAATAAC-3′, inner antisense 5′-GGTTGTAGTCATTCTCGTCCAGGG-3′), which generates a 115-bp PCR product.
The amplified products were transferred onto nitrocellulose paper and subjected to Southern blot hybridization with a 25-bp internal probe end-labeled with [32P]-deoxycytidine triphosphate, which represents the HHV-8 sequence of bp 1078 to 1102, as described previously (Chang et al, 1994).
ISH for HHV-8
For ISH studies, a 115-bp DNA fragment of the KSHV ORF72 was amplified by PCR of DNA obtained from biopsy of proven KS of the pleura (Gómez-Román et al, 1999). ORF72 encodes a putative protein of 257 amino acids that shows homology to ORF72 of herpesvirus saimiri encoding a cyclin D homolog and to multiple mammalian cyclin D proteins (Cesarman et al, 1996). Probes were generated using primers P51 (5′ primer: 5′-CACCCTGAAACTCCAGGC-3′) and P32 (3′ primer: 5′-GATCCGATCCTCACATAGCG-3′). The PCR product was purified (CONCERT Rapid PCR Purification System; Life Technologies, Barcelona, Spain) and analyzed by electrophoresis on a 1% agarose gel (data not shown). The purified PCR product was biotinylated (BioPrime DNA Labeling System; Life Technologies) following the manufacturer's instructions. The biotinylated probe was purified by gel filtration using Sephadex G50 columns (Amersham-Pharmacia, Madrid, Spain). To determine the suitability of the tissue sections, we used a commercial biotinylated probe to total genomic DNA of cultured human cells as a positive control (DAKO Corporation, Carpinteria, California). A negative-control probe (plasmid DNA) was prepared from the plasmid vector PCR 2.1 (Invitrogen, San Diego, California). We also used additional probes raised against sequences of human papillomavirus as negative controls.
ISH was performed in 5-μm thick histopathologic sections. After deparaffination and rehydration through a graded ethanol series, slides were immersed in 3% hydrogen peroxide in methanol for 10 minutes to block the endogenous peroxidase activity and washed with distilled water. Proteinase K solution (10 μg/ml) was applied to each section and slides were incubated (37° C, 30 minutes) in a water bath humidity chamber. After a further washing step, the KSHV-specific biotinylated probe (ORF72) was diluted to a concentration of 0.1 μg/ml in a solution containing: 10% v/v 20x SSC, 10% v/v Denhardt's solution, 2.5% salmon testes DNA (10 mg/ml), 50% v/v deionized formamide, 22.5% v/v double-distilled water with 10% w/v dextran sulfate, and 5% v/v yeast tRNA (10 ng/ml). Fifty microliters of this probe cocktail was applied to each slide, covered with a glass coverslip, heated (95° C, 5 minutes) on a hotplate to denature both probe and target sequence, and incubated (37° C, 16 hours) in a humidified chamber to allow specific hybridization. After hybridization, coverslips were gently removed by soaking slides in 0.05 mol/l of Tris-buffered saline (TBS), pH 7.4, followed by 0.2% SDS. The slides were washed in TBS and rinsed in TBS-Tween (20 μl of Tween per liter of TBS) for 5 minutes.
To detect the ISH signal, we used a nonisotopic, colorimetric tyramide signal amplification system (DAKO GenPoint; DAKO Corporation) according to the manufacturer's recommendations. Positive controls consisted of skin biopsies from several patients with cutaneous KS. Negative controls consisted of cultured cells of an acute myelogenous leukemia (HL-60) that did not contain virus, and skin biopsies from otherwise healthy individuals. Counterstaining was performed with Harris hematoxylin and interpreted in a blinded fashion by two independent pathologists.
Immunohistochemistry
The mouse monoclonal antibodies used for immunohistochemistry were: IgG1 anti-recombinant human IL-6 (monoclonal, diluted 1:100; Genzyme, Cambridge, Massachusetts); IgG2a anti-vimentin (O15-D, prediluted; Biomeda, Foster City, California); α-smooth muscle actin (1A4, diluted 1:50; DAKO, Glostrup, Denmark); IgG2a anti-human low molecular weight cytokeratins (CAM5.2, prediluted; Becton-Dickinson, San Jose, California); IgG1 anti-human high molecular weight cytokeratins (34βE12, diluted 1:40; DAKO); and IgG1 anti-human cyclin D1 (DCS-6, diluted 1:100; DAKO). Immunohistochemistry was performed using formalin-fixed, paraffin-embedded tissue sections. The IL-6 antibody was incubated overnight at 4° C. Antigen retrieval was performed by boiling sections in citric acid buffer in a pressure cooker for 90 seconds for all antibodies, except for the cyclin D1 antibody, for which 0.1 mm EDTA, pH 8.0, buffer was used. The Dako EnVision + kit was used as a visualization system according to the manufacturer's instructions, in a Techmate 500–220 automated immunostainer (Biotek, Santa Barbara, California) (Vyberg and Nielsen, 1998). Diaminobenzidine was used as the chromogen.
References
Arber DA, Weiss LM, and Chang KL (1998). Detection of Epstein-Barr virus in inflammatory pseudotumor. Sem Diagn Pathol 15:155–160.
Cesarman E and Knowles DM (1997). Kaposi's sarcoma-associated herpesvirus: A lymphotropic human herpesvirus associated with Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Sem Diagn Pathol 14:54–66.
Cesarman E, Nador RG, Bai F, Bohenzky RA, Russo JJ, Moore PS, Chang Y, and Knowles DM (1996). Kaposi's sarcoma associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi's sarcoma and malignant lymphoma. J Virology 70:8218–8223.
Coffin CM, Dehner LP, and Meis-Kindblom JM (1998a). Inflammatory myofibroblastic tumor, inflammatory fibrosarcoma, and related lesions: An historical review with different diagnostic considerations. Sem Diagn Pathol 15:102–110.
Coffin CM, Humphrey PA, and Dehner LP (1998b). Extrapulmonary inflammatory myofibroblastic tumor: A clinical and pathological survey. Sem Diagn Pathol 15:85–101.
Chang Y, Cesarman E, Pessin MS, Lee F, Culpepper J, Knowles DM, and Moore PS (1994). Identification of herpesvirus-like sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865–1869.
Chang J, Renne R, Dittmer D, and Ganem D (2000). Inflammatory cytokines and the reactivation of Kaposi's sarcoma-associated herpesvirus lytic replication. Virology 266:17–25.
DiAlberti L, Piatelli A, Artese L, Favia G, Patel S, Saunders N, Porter SR, Scully CM, Ngui SL, and Teo CG (1997). Human herpesvirus 8 variants in sarcoid tissues. Lancet 350:1655–1661.
Foreman KE, Bacon PE, Hsi ED, and Nickoloff BJ (1997). In situ polymerase chain reaction-based localization studies support role of human herpesvirus-8 as the cause of two AIDS-related neoplasms: Kaposi's sarcoma and body cavity lymphoma. J Clin Invest 99:2971–2978.
Gallo RC (1998). The enigmas of Kaposi's sarcoma. Science 282:1837–1839.
Ganem D (1997). KSHV and Kaposi's sarcoma: The end of the beginning? Cell 91:157–160.
Gilison ML and Ambinder RF (1997). Human herpesvirus-8. Current Opinion in Oncology 9:440–449.
Gómez-Román JJ, Ocejo-Vinyals JG, Sánchez-Velasco P, Leyva-Cobián F, and Val-Bernal JF (1999). Presence of Human Herpesvirus 8 DNA sequences in renal transplantation-associated pleural Kaposi's Sarcoma. Arch Pathol Lab Med 123:1269–1273.
Kishimoto T, Akira S, and Taga T (1992). Interleukin-6 and its receptor: A paradigm for cytokines. Science 258:593–597.
Lin BTY, Chen YY, Battifora H, and Weiss LM (1996). Absence of Kaposi's sarcoma-associated herpesvirus-like DNA sequences in malignant vascular tumors of the serous membranes. Mod Pathol 9:1143–1146.
Marchioli CC, Love JL, Abbott LZ, Huang YQ, Remick SC, Surtento-Reodica N, Hutchison RE, Mildvan D, Friedman-Kien AE, and Poiesz BJ (1996). Prevalence of human herpesvirus-8 DNA sequences in several patient populations. J Clin Microbiol 34:2635–2638.
Matsubara O, Tan-Liu NS, Kenney RM, and Mark EJ (1988). Inflammatory pseudotumors of the lung: Progression from organizing pneumonia to fibrous histiocytoma or to plasma cell granuloma in 32 cases. Hum Pathol 19:807–814.
Memar OM, Rady PL, Goldblum RM, and Tyring SK (1997). Human herpesvirus-8 DNA sequences in a patient with pemphigus vulgaris but without HIV infection or Kaposi's sarcoma. J Invest Dermatol 108:118–119.
Moore PS, Boshoff C, Weiss R, and Chang Y (1996a). Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science 274:1739–1744.
Moore PS, Kingsley LA, Holmberg SD, Spira T, Gupta P, Hoover DR, Parry JP, Conley LJ, Jaffe JW, and Chang Y (1996b). Kaposi's sarcoma-associated herpesvirus infection prior to onset of Kaposi's sarcoma. AIDS 10:175–180.
Parravicini C, Corvbellino M, Paulli M, Magrini U, Lazzarino M, Moore PS, and Chang Y (1997). Expression of a virus-derived cytokine, KSHV vIL-6 in HIV-seronegative Castleman's disease. Am J Pathol 151:1517–1522.
Rohrlich P, Peuchmaur M, de Napoli S, Durand I, Garel C, Aigrain Y, Galanaud P, Vilmer E, and Emilie D (1995). Interleukin-6 and Interleukin-1β production in a pediatric plasma cell granuloma of the lung. Am J Surg Pathol 19:590–595.
Russo JJ, Bohenzky RA, Chien MC, Chen J, Yan M, Maddalena D, Parry JP, Peruzzi D, Edelman IS, Chang Y, and Moore PS (1996). Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8). Proc Natl Acad Sci USA 93:14862–14867.
Sun R, Lin SF, Staskus K, Gradoville L, Grogan E, Haase A, and Miller G (1999). Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J Virol 73:2232–2242.
Trovato R, Luppi M, Barozzi P, Da Prato L, Maiorana A, Lico S, Marasca R, Torricelli P, Torelli G, and Ceccherini-Nelli L (1999). Cellular localization of human herpesvirus 8 in nonneoplastic lymphadenopathies and chronic interstitial pneumonitis by in situ polymerase chain reaction studies. J Hum Virol 2:38–44.
Vyberg M and Nielsen S (1998). Dextran polymer conjugate two-step visualization system for immunohistochemistry. A comparison of EnVision plus with two three stepavidin-biotin techniques. Appl Immunohistochem 6:3–10.
Wan X, Wang H, and Nicholas J (1999). Human herpesvirus 8 interleukin-6 (vIL-6) signals through gp130 but has structural and receptor-binding properties distinct from those of human IL-6. J Virol 73:8268–8278.
Weiss SW (1994). Histological typing of soft tissue tumors, 2nd ed. Springer-Verlag, New York:48.
Acknowledgements
The authors thank Dr. F. Arce (Hospital “Marqués de Valdecilla,” Santander) and Dr. I. M. Outschoorn (Instituto Carlos III, Madrid) for critical review of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was partially supported by grants from the Spanish Ministry of Health (FIS 97/1119) and the Fundación “Marqués de Valdecilla.” The Fundación “Marcelino Botín” supported equipment costs (genetic analyzer and PCR systems) at the Department of Immunology.
The contents of this work have been partially presented in EUROCELLPATH'99 in Paris, France, June 24–28, 1999.
Rights and permissions
About this article
Cite this article
Gómez-Román, J., Ocejo-Vinyals, G., Sánchez-Velasco, P. et al. Presence of Human Herpesvirus-8 DNA Sequences and Overexpression of Human IL-6 and Cyclin D1 in Inflammatory Myofibroblastic Tumor (Inflammatory Pseudotumor). Lab Invest 80, 1121–1126 (2000). https://doi.org/10.1038/labinvest.3780118
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.3780118
This article is cited by
-
Ein seltener inflammatorisch myofibroblastärer Tumor der Lunge
Zeitschrift für Herz-,Thorax- und Gefäßchirurgie (2016)
-
Vaginal superficial myofibroblastoma: a rare mesenchymal tumor of the lower female genital tract and a study of its association with viral infection
Medical Molecular Morphology (2012)
-
Absence of human herpesvirus-8 in pulmonary inflammatory myofibroblastic tumor: immunohistochemical and molecular analysis of 20 cases
Modern Pathology (2007)
-
Pulmonary “inflammatory myofibroblastic” tumors: a critical examination of the diagnostic category based on quantitative immunohistochemical analysis
Virchows Archiv (2007)
-
Pulmonary Inflammatory Myofibroblastic Tumor Resected by Video-Assisted Thoracoscopic Surgery: Report of a Case
Surgery Today (2007)