Abstract
Characterization of endogenous synthesis of prolactin (PRL) proteins and their cellular localization in labial salivary glands of patients with Sjögren's syndrome (SS) were achieved. PRL, PRL-receptors (PRL-R), and S100A6 protein were detected by immunohistochemistry. In situ prolactin synthesis was investigated in controls and SS patients by ex vivo incubation of minor salivary glands biopsies and immunoprecipitation assay. Increased PRL-immunoreactivity was found in cytoplasmic acinar epithelial cells in SS patients compared with normal subjects. PRL-R was distributed only in ductal epithelial cells in which S100A6 protein (a PRL-R-associated protein) was also present. PRL, PRL-R, or S100A6-immunoreactivity was not detected in infiltrating mononuclear cells. Immunoprecipitation demonstrated that PRL synthesis occurred in minor salivary glands with increased synthesis of two distinct PRL-like proteins (one major band at 60 kDa and a minor at 16 kDa) in SS glands compared with normal glands. Expression of PRL gene was demonstrated in SS salivary glands using RT-PCR. A positive correlation was found between the presence of PRL-like proteins in acinar epithelial cells of SS patients and clinical extraglandular manifestations. The presence of anti-Ro and anti-La antibodies also positively correlated with a higher percentage of PRL in acinar epithelial cells. In conclusion, PRL-like proteins are synthetized and overexpressed in glandular epithelial cells of labial salivary glands from SS patients and correlate with the aggressiveness of the disease.
Similar content being viewed by others
Introduction
Sjögren's syndrome (SS) is an autoimmune disorder characterized by lymphocytic infiltration into salivary and lacrimal glands clinically leading to dry eyes (keratocunjunctivitis sicca) and dry mouth (xerostomia). This condition is one of the more common autoimmune diseases, with a prevalence of 1% to 3% (Dafni et al, 1997). SS may be primary when isolated or secondary when associated with another autoimmune disease, most frequently rheumatoid arthritis or systemic lupus erythematosus (Dafni et al, 1997). The precise mechanism responsible for the impairment of the glandular tissue remains unknown, but a direct lymphocyte-glandular epithelial cell contact might be involved with infiltrating T-lymphocytes exhibiting a CD4+αβCD45Ro phenotype (Adamson et al, 1983) that can initiate glandular cell apoptosis through granzyme A and perforin production (Alpert et al, 1994; Moutsopoulos, 1994).
The epithelial cell is not an innocent bystander, but plays a major role in the induction and perpetuation of the autoimmune reaction (Polihronis et al, 1998). Different factors implied in such reaction were found, such as overexpression of HLA DR molecules (Fox et al, 1986), proinflammatory cytokines (Fox et al, 1998), oncogenes (Skopouli et al, 1992), and autoantigens (Warhen et al, 1998).
Among the cytokines of interest, prolactin (PRL) seems to be one closely linked to autoimmune diseases. The peptide hormone prolactin belongs to the cytokine superfamily of the helix bundle peptide hormones (Goffin et al, 1996) and its receptors are genetically a part of the cytokine-hematopoietin receptor superfamily (Cosman, 1993). PRL is known to stimulate humoral and cell-mediated immune responses and may have a role in the pathogenesis of various autoimmune diseases, including systemic lupus erythematosus (Jimena et al, 1998; Neidhart, 1996). Furthermore, hyperprolactinemia was found in 46% of patients with primary Sjögren's syndrome (Gutiérrez et al, 1994), correlation between high levels of prolactin and active disease has been observed (Allen et al, 1996), and serum PRL was recently associated with extraglandular disease manifestations in primary SS (Haga and Rygh, 1999).
In this work, we investigated whether PRL is expressed in labial salivary glands (LSG) of patients with SS. We thus immunohistochemically looked for the presence and the tissue localization of PRL, Prolactin-receptor (PRL-R), and S100A6 protein, a PRL-R-associated-protein (Murphy et al, 1988), in the LSG from SS patients and from normal subjects, and investigated whether salivary glandular epithelial cells synthetized and differentially expressed PRL subtypes in controls and patients with SS.
Results
Characterization of PRL, PRL-Receptors, and S100A6 Protein in Labial Salivary Glands of SS and Control Subjects
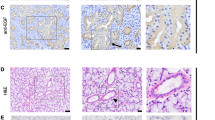
Prolactin immunoreactivity was prominently detected in acinar (22.9 ± 4.8%) (Table 1) and ductal (24.3 ± 7.1%) epithelial cells of SS labial salivary glands (Fig. 1, A, G to H), whereas the percentages were lower in control acinar (2.7 ± 1.4%) and similar (21.7 ± 11.4%) in ductal epithelial cells (Fig. 1B). The staining intensity was slightly but significantly increased (p = 0.04) in the acinar cells of SS compared with controls.
Immunohistochemistry of human salivary glands. Localization of prolactin (PRL), prolactin receptor (PRL-R), and S100A6 protein in labial salivary gland (LSG) biopsies from patients with Sjögren's syndrome (SS) (A, C, and E, respectively) and controls (B, D, and F, respectively). A and G to H illustrate PRL immunoreactivity visible in the acinar epithelial cells of LSG from SS compared with controls (B). C and D show that PRL-R was only detected in ductal epithelial cells from SS patients and poorly in controls. E and F show S100A6 protein immunoreactivity in ductal epithelial cells only in SS minor salivary glands. Note the S100A6 staining in nervous tissue (arrowheads). The right half of G shows a mononuclear infiltrate negative for PRL; the left half shows PRL positive epithelial cells (original magnification, × 250). H shows PRL negative lymphocytes (arrows) at a higher magnification (original magnification, × 400).
PRL-R immunoreactivity was primarily localized in the cytoplasm (Fig. 1, C to D). The PRL-R was present in ductal epithelial cells only, from SS (65.3 ± 8.9%) and controls (46.5 ± 14.3%), with no statistical difference (p > 0.05) between these two groups.
Although the S100A6 protein was absent from acinar epithelial cells, it was highly expressed in ductal epithelial cells (80.9 ± 8.5%) of SS biopsies (Fig. 1E), but significantly (p = 0.03) lower in controls (34.5 ± 13.9%). Furthermore, the ductal S100A6 staining intensity clearly increased in SS compared with controls (p = 0.004). S100A6 immunoreaction was significantly and positively correlated (p = 0.01) with PRL-R staining.
Infiltrating mononuclear cells were not immunoreactive for PRL in LSG from SS patients (Fig. 1, G to H), as well as for PRL-R and S100A6 protein (Fig. 1, C and E).
Synthesis of Prolactin-Like Proteins in Glandular Epithelial Cells of SS Patients
A Western blot using homogenates prepared with biopsies obtained from ten different patients with primary SS indicated the presence of 60-kDa PRL (data not shown). Three paired (one from SS and one from a normal subject) labial salivary gland biopsies were used to visualize protein synthesis and, after immunoprecipitation, PRL synthesis. The total amount of aminoacids incorporated into proteins, as measured by TCA precipitation, was comparable in each paired biopsies (not shown). Thus, in our experimental conditions, no significant difference in overall protein synthesis could be seen between SS and normal salivary glands. Immunoprecipitation, electrophoresis, and autoradiography of the neosynthetized proteins revealed a major band of proteins around 60 kDa and another band at 16 kDa in the SS glands, whereas barely detectable radioactive bands were seen in immunoprecipitates from normal glands (Fig. 2). To verify the specificity of the antibody, immunoprecipitation of human pituitary was also performed. Two bands were obtained, one at 22 kDa, corresponding to pituitary PRL, and the other at 60 kDa (not shown).
Synthesis of prolactins by human salivary gland from SS patients and normal subjects. Salivary glands were metabolically labeled in the presence of 35S-cysteine and 35S-methionine for 8 hours. Radiolabeled proteins from the tissue homogenates were immunoprecipitated with human anti-prolactin antibody. The positions of molecular mass markers are indicated by arrows. CT, control subject; SS, Sjögren's syndrome patients.
Expression of Prolactin Gene
Figure 3 shows the presence of prolactin transcripts in the four SS labial salivary glands (lanes 2, 4, 8, and 9) but not in the five healthy controls (lanes 1, 3, 5, 6, and 7). The lanes 10, 11, and 12 are positive controls for the expression of the prolactin gene, respectively two pituitary glands (lanes 10 and 11) and human normal brain cDNA (lane 12).
RT-PCR analysis of prolactin mRNA expression. Lanes 2, 4, 8, and 9 are labial salivary glands from four patients with SS; lanes 1, 3, 5, 6, and 7 are labial salivary glands from five healthy controls; lanes 10, 11, and 12 are positive controls for the expression of PRL gene, respectively two pituitary glands (lanes 10 and 11) and human normal brain cDNA (lane 12).
Relation Between Prolactin Expression and Clinical Manifestations
Association between the presence of PRL in acinar cells and clinical extraglandular manifestations was made. Three groups defined as normal, SS with, and without extraglandular manifestations were analyzed. The results show that PRL-immunoreactivity in acinar epithelial cells of labial salivary glands was 2.7 ± 1.4% in normal group, 6.2 ± 3.2% in SS without, and 40.2 ± 8.1% with extraglandular manifestations (p = 0.001) (Fig. 4A).
Correlation between acinar prolactin expression and clinical data. Representation of the mean (small squares) ± the standard error (large rectangles) ± the standard deviation (bars) values of the percentage of prolactin-stained salivary acinar cells in A. A, Normal subjects (CT), SS with extraglandular manifestations (SS+EG), SS without extraglandular manifestations (SS-EG); B, in the presence of anti-SS-A (SS-A) and anti-SS-A with anti-SS-B antibodies (SS-A+SS-B).
Relation Between Prolactin Expression and Antinuclear Autoantibodies
PRL expression in the acinar epithelial cells of SS salivary glands increased significantly (p = 0.04) with the presence of anti-Ro/SS-A and anti- La/SS-B antibodies (Fig. 4B). Four of 13 patients in the secondary SS group have positive antinuclear antibodies without identification of anti-Ro/SS-A or anti-La/SS-B. In these four patients, almost no PRL positive cells were found.
Discussion
The present study shows that big prolactin is overexpressed in labial salivary glands from patients with SS. Two prolactin proteins were identified, ie, a major band at approximately 60 kDa and second at 16 kDa. Although located on one single gene coding for a 21,500 Dalton single chain polypeptide, prolactin may occur in 60 kDa (big prolactin) (Von Werder and Clemm, 1974) and 150 kDa (big-big prolactin) (Larrea et al, 1987). Big prolactin is able to bind concanavalin A (Shoupe et al, 1983) and therefore is a glycosylated form. Incidentally, if big prolactin is a glycosylated form of the single prolactin polypeptide chain, approximately 180 sugar moieties (hexoses) should be covalently linked to the 227 PRL aminoacids, which does not seem to be of steric hindrance for epitope recognition by the monoclonal antibody raised against the native 22-kDa prolactin. Production of big prolactin has already been associated with other autoimmune diseases, particularly with systemic lupus erythematosus. In these patients, big prolactin is produced in peripheral blood mononuclear cells (Larrea et al, 1997). In our study the cell types expressing big prolactin in SS patients is different. Why there is such a difference in the tissue expression is not known, but big prolactin expression has previously been observed in activated peripheral blood mononuclear cells (Sabhrawal et al, 1992), as well as in vascular and epithelial tissues (Wu et al, 1996). More interesting is the close genomic linkage between HLA alleles and the prolactin gene. Indeed, human prolactin gene is located on chromosome 6 (Owerbach et al, 1981), more particularly in the interval 6p22.2 to p21.3 (Evans et al, 1989) close to specific HLA alleles. This regional assignment is of interest because a recent study (Brennan et al, 1997) presented data that there may be linkage disequilibrium between HLA-DRB1 alleles and microsatellite marker close to the prolactin gene among women with rheumatoid arthritis and systemic lupus erythematosus. Such genetic susceptibility has also been described in primary SS (Guggenbuhl et al, 1998; Jean et al, 1998). Taken together, our data give further substance to the hypothesis that high prolactin production is linked to autoimmune diseases, including Sjögren's syndrome.
We also demonstrated that synthesis of a 16-kDa prolactin slightly increased in SS patients. 16-kDa prolactin is the result of the enzymatic cleavage of 22-kDa prolactin (Mittra, 1980), and it has recently been found that the 16-kDa compound has an anti-angiogenic effect (Struman et al, 1999). The presence of PRL receptors was shown in striated duct cells using either anti-PRL-receptor or anti-S100A6 antibodies. The antibody we used could not distinguish between the long or short form of the PRL-receptors family. The calcium-binding S100A6 protein (also named calcyclin) is an associated PRL-receptor protein, which like PRL binds Zn2+ (Heizmann and Cox, 1998), was detected in ductal cells from human salivary glands (Huang et al, 1996) and stimulated in vitro secretion of mouse placental lactogen (Farnsworth and Talamantes, 1998). We therefore used anti-S100A6 antibody against PRL-receptor and obtained a good correlation between S100A6 localization and PRL receptors. The cytoplasmic nature of the PRL-R labeling is in accordance with observation by others (Garcia-Caballero et al, 1996) and may be explained by the rapid internalization of the PRL-R complexes by endocytosis (Genty et al, 1994). Our results thus suggest a correlation between the presence of big prolactin and prolactin receptors, but further experiments are to be made to demonstrate if big prolactin is indeed interacting with prolactin receptors and able to activate or inhibit the receptors.
Interestingly, the presence of anti-Ro/SS-A and anti-La/SS-B antibodies was associated with a higher percentage of prolactin in acinar epithelial cells of SS labial salivary glands. Hyperprolactinemia was reported to be associated, in young women (< 50 years old), with a higher incidence of autoantibodies, including anti-dsDNA and anti-Ro/SS-A; and in women of > 50 years, with anti-Ro/SS-A and anti-La/SS-B (Allen et al, 1996). This finding enhanced the hypothesis that PRL may induce the production of autoantibodies (Gutiérrez et al, 1996). Finally, our observation that association between elevated expression of PRL in the salivary acinar cells of SS patients and clinical extraglandular manifestations, which is in agreement with Haga and Rygh (1999), led us to propose that big PRL can be a clinical sign of a more aggressive disease. However, the exact clinical significance of high PRL levels in acinar epithelial cells of labial salivary glands from SS patients needs to be clarified through longitudinal studies.
Materials and Methods
Patients and Controls
Twenty-six patients with a diagnosis of SS without evidence of endocrine disease were studied. All patients fulfilled both the San Diego (Fox et al, 1986a) and the modified European Community (Vitali et al, 1993) classification criteria for SS. Thirteen patients have primary SS (12 women, 1 man; aged 25 to 75 years, mean 56), whereas 13 have secondary SS (10 women, 3 men; aged 20 to 75 years, mean 51). All of the secondary SS were associated with rheumatoid arthritis. Fifteen healthy volunteers (14 women, 1 man; aged 22 to 67 years, mean 43) were studied as controls. The patients' characteristics are summarized in Table 2. None of the SS or control subjects were receiving medication known to be associated with increased PRL secretion, or had documented hypothyroidism at the start of the study. Peripheral neuropathy was establish on the basis of symptoms, clinical sensory deficits, and electrophysiologic findings, and was further confirmed by a neurologist. Lung involvement included dry cough and obstructive airways disease (based on pulmonary function tests) and one case of lymphocytic interstitial pneumonitis. {tabft}a Pulmonary involvement = dry cough, obstructive airways disease, and lymphocytic interstitial pneumonitis; b Sensory neuropathy was defined on clinical and electrophysiological testing.
Determination of Antinuclear Antibodies
An indirect immunofluorescence procedure using Hep0.2 cell substrates was employed to detect the presence and titer of antinuclear antibody (ANA; Immunoconcept, Sacramento, California). Anti-SSA/Ro and anti SSB/La antibodies were detected by means of ELISA (INOVA Diagnostics, San Diego, California).
Immunohistochemical Staining
After informed consent was obtained from each subject under study, labial salivary gland tissue biopsies were performed in patients with SS and in normal volunteers. Immediately after removal, tissue specimens were fixed in 4% buffered formaldehyde and embedded in paraffin. Sections 5 μm thick taken from each specimen were subjected to processing with the different histochemical probes and kit reagents under study: anti-mouse IgG1 monoclonal antibody (Mabs) against human prolactin, diluted to 1:100 (BioGenex, San Ramon, California); Mabs against prolactin-receptors (1:100 dilution; Affinity Bioreagents, Golden, Colorado); goat antibodies against recombinant human S100A6 protein (1:10,000 dilution). Incubation with labeled probes was carried out at 25 ± 1° C for 60 minutes (PRL and PRL-R) or 120 minutes (S100A6 protein). The extent of the specifically bound biotinylated probes was visualized by avidin-biotin-peroxidase complex (ABC) kit reagents (Vector Labs, Burlingame, California), with diaminobenzidine/H2O2 as the chromogenic substrate. Two types of controls were performed: the first control included the omission of the incubation step with the primary antibody to verify the absence of any staining by unspecific binding of reagents, horseradish peroxidase, and avidin; and the second control included the preincubation of the respective antibodies (at their working dilutions) with 1 μg/ml of pure hPRL (Sigma-Aldrich, Belgium) and recombinant human S100A6 (Ilg et al, 1996). Both controls gave no staining (data not shown). Counterstaining with Toluidine blue concluded the routine.
Immunoprecipitation Assay
Labial salivary gland biopsies from SS patients and normal subjects were collected within the same hour, immersed in medium, and further incubated for 1 hour in methionin- and cystein-free medium (DMEM; ICN Biomedicals, Irvine, California) at 37° C in the CO2 incubator. Tissues were labeled with 0.1 mCi/ml of TRAN35S-LABEL (1175 Ci/mmol; ICN) for 8 hours at 37° C in the same medium. The tissue samples were washed three times with cold PBS and homogenized in a Teflon glass homogenizer using 500 μL RIPA lysis buffer that included 0.1 m Tris HCl, pH 7.4, 0.15 m NaCl, 1 mm PMSF, 0.1% (w/v) SDS, 1 mm EDTA, 1% (w/v) Triton X-100, 0.5% (w/v) aprotinin, and 0.5% (w/v) sodium deoxycholate (Cheresh and Spiro, 1987). Homogenates were centrifuged at 3,000 g for 30 minutes at 4° C and the pellets discarded. Supernatants were loaded onto 40 μl of Protein G Sepharose 4 Fast Flow (Pharmacia Biotech, Upsala, Sweden). After addition of monoclonal anti-human prolactin (Biogenex, San Ramon, California) (1:60 dilution) and incubation overnight at 4° C, the precipitated immune complexes were washed with PBS 0.1% Triton X-100, PBS 0.5 mm NaCl, and PBS before dilution in 40 μl of Laemmli buffer. Samples were analyzed by electrophoresis on 12% SDS PAGE under reducing conditions. Radiolabeled immunoprecipitated proteins were visualized by autoradiography. Control immunoprecipitation was made using no antibody to verify the absence of unspecific immunoglobulins retained on protein G sepharose beads in SS (data not shown). The total amount of radioactive amino-acid incorporated into proteins was estimated by TCA precipitation obtained from whole homogenate. This control allowed us to count and verify that normal and SS tissues had incorporated the same amount of radioactivity into the proteins. Three independent sets of experiments gave the same results.
Determination of Immunohistochemical Staining
A semi-quantitative estimation of the immunohistochemical staining was carried out as detailed previously for lectin histochemistry in meningiomas (Salmon et al, 1996). This method was based on a distinct estimation of both the staining intensity (SI) of each probe and the relative abundance of immunoreactive cells (labeling index, LI) in the acinar and ductal epithelial cells and infiltrating mononuclear cells of labial salivary gland biopsies. The LI variable corresponded to the percentage of positive cells in at least 20 fields (original magnification, × 25) per specimen. Positive cells in each section were counted field by field. The percentage of positive cells of each cell type compared with the total number of cells of that type in the sections was determined. The SI variable (assessed in the histologic fields used for the assessment of the LI variable) was graded 0 (absence of staining or staining equivalent to background staining in the negative control), 1 (weak to moderate staining), and 2 (intense staining).
Reverse Transcription and PCR
Total RNA from control and SS labial salivary glands was extracted and a DNase treatment performed using the S.N.A.P. Total RNA Isolation kit (Invitrogen, Carlsbad, California). Reverse Transcription of 750 ng of total RNA was performed with the Superscript II kit (GIBCO BRL Life Technologies). PCR were performed on cDNA samples for prolactin and glyceraldehyde–3–phosphate– dehydrogenase (GAPDH), respectively, using as forward primers: 5′-GCCATCAACAGCTGCCACACT3′, 5′CGGA-GTCAACGGATTTGGTCGTAT-3′, and as reverse primers: 5′-GACTCTTCATCAGCCATCTGC-3′, 5′-AGCCTT-CTCCATGGTGGTGAAGAC-3′. All PCR were performed with the Taq PCR Core kit (Qiagen, Leusden, The Netherlands) on 1/10 of the volume of the room temperature reaction and 100 ng of forward and reverse primers, in a Stratagene Robocycler Gradient 96 thermocycler (Stratagene, La Jolla, California) using the following conditions: 93° C for 3 minutes; 93° C for 1 minute, 57° C for 1 minute, 72° C for 1 minute, 35 cycles; 72° C for 6 minutes; rapid cooling to 4° C. Amplified products (332 bp for prolactin, 306 bp for GAPDH) were subjected to electrophoresis on a 2% agarose gel stained with ethidium bromide.
Statistical Analysis
The Mann-Whitney test (for comparing two groups) and the Kruskall-Wallis test (for comparing more than two groups) were used to evaluate the statistical differences between the histoclinical groups (normal, primary SS, and secondary SS) and each probe expression (distinctly estimated in the acinar, ductal epithelial cells, and mononuclear cells by means of both the LI and SI variables). Correlation was revealed by means of the Kendall Tau test. All of the statistical analyses were carried out using the Statistica software (Statsoft, Tulsa, Oklahoma).
References
Adamson TCIII, Fox RI, Frisman DM, and Howell FV (1983). Immunohistologic analysis of lymphoid infiltrates in primary Sjögren's syndrome using monoclonal antibodies. J Immunol 130:203–208.
Allen SH, Sharp GC, Wang G, Conley C, Takeda Y, Conroy SE, and Walker SE (1996). Prolactin levels and antinuclear antibody profiles in women tested for connective tissue disease. Lupus 5:30–37.
Alpert S, Kang HI, Weissman I, and Fox RI (1994). Expression of granzyme A in salivary gland biopsies from patients with primary Sjögren's syndrome. Arthritis Rheum 37:1046–1054.
Brennan P, Hajeer A, Ong KR, Worthington J, John S, Thomson W, Silman A, and Ollier B (1997). Allelic markers close to prolactin are associated with HLA-DRB1 susceptibility alleles among women with rheumatoid arthritis and systemic lupus erythematosus. Arthritis Rheum 40:1383–1386.
Cheresh DA and Spiro RC (1987). Biosynthetic and functional properties of an Arg-Gly-Asp-directed receptor involved in human melanoma cell attachment to vitronectin, fibrinogen, and von Willebrand factor. J Biol Chem 262:17703–17711.
Cosman D (1993). The hematopoietin receptor superfamily. Cytokine 5:95–106.
Dafni UG, Tzioufas AG, Staikos P, Skopouli FN, and Moutsopoulos HM (1997). Prevalence of Sjögren's syndrome in a closed rural community. Ann Rheum Dis 56:521–525.
Evans AM, Petersen JW, Sekhon GS, and DeMars R (1989). Mapping of prolactin and tumor necrosis factor-beta genes on human chromosome 6p using lymphoblastoid cell deletion mutants. Somat Cell Mol Genet 15:203–213.
Farnsworth RL and Talamantes F (1998). Calcyclin in the mouse decidua: Expression and effects on placental lactogen secretion. Biol Reprod 59:546–552.
Fox RI, Bumol T, Fantozzi R, Bone R, and Schreiber R (1986). Expression of histocompatibility antigen HLA-DR by salivary gland epithelial cells in Sjögren's syndrome. Arthritis Rheum 29:1105–1111.
Fox PC, Grisius MM, Bermuez DK, and Sun D (1998). Cytokine mRNA expression in labial salivary glands and cytokine secretion in parotid saliva in Sjögren's syndrome. Adv Exp Med Biol 438:909–915.
Fox RI, Robinson CA, Curd JG, Kozin F, and Howell FV (1986a). Sjögren's syndrome. Proposed criteria for classification. Arthritis Rheum 29:577–585.
Garcia-Caballero T, Morel G, Gallego R, Fraga M, Pintos E, Gago D, Vonderhaar BK, and Beiras A (1996). Cellular distribution of prolactin receptors in human digestive tissues. J Clin Endocrinol Metab 8:1861–1886.
Genty N, Paly J, Edery M, Kelly PA, Djiane J, and Salesse R (1994). Endocytosis and degradation of prolactin and its receptor in Chinese hamster ovary cells stably transfected with prolactin receptor cDNA. Moll Cell Endocrinol 99:221–228.
Goffin V, Shiverick KT, Kelly PA, and Martial JA (1996). Sequence-function relationships within the expanding family of prolactin, growth hormone, placenta lactogen and related proteins in mammals. Endocrinol Rev 17:385–410.
Guggenbuhl P, Jean S, Jego P, Grosbois B, Chalès G, Semana G, Lancien G, Veillard E, Pawlotsky Y, and Perdriger A (1998). Primary Sjögren's syndrome: Role of the HLA-DRB1*0301-*1501 heterozygotes. J Rheumatol 25:900–905.
Gutiérrez MA, Anaya JM, Scopelitis E, Citera G, Silveiro LH, and Espinoza LR (1994). Hyperprolactinemia in primary Sjögren's syndrome (Letter). Ann Rheum Dis 53:425–428.
Gutiérrez MA, Molina JF, Jara LJ, Garcia C, Gutiérrez-Urena S, Cuellar MC, Gharavi A, and Espinoza LR (1996). Prolactin-induced immunoglobulin and autoantibody production by peripheral blood mononuclear cells from systemic lupus erythematosus and normal individuals. Int Arch Allergy Immunol 109:229–235.
Haga HJ and Rygh T (1999). Prevalence of hyperprolactinemia in patients with primary Sjögren's syndrome. J Rheumatol 26:1291–1295.
Heizmann CW and Cox JA (1998). New perspectives on S100 proteins: A multi-functional Ca(2+)- Zn(2+)- and Cu(2+)-binding protein family. BioMetals 1:383–397.
Huang JW, Ming Z, Shrestha P, Mori M, Ilg E, Schäfer BW, and Heizmann CW (1996). Immunohistochemical evaluation of the Ca(2+)-binding S-100 proteins S-100A1, S-100A2, S-100A4, S-100A6 and S-100B in salivary gland tumors. J Oral Pathol Med 25:547–555.
Ilg EC, Schäfer BW, and Heizmann CW (1996). Expression pattern of S100 calcium-binding proteins in human tumors. Int J Cancer 68:325–332.
Jean S, Quelvennec E, Alizadeh M, Guggenbuhl P, Birebent B, Perdriger A, Groibois B, Pawlotsky PY, and Semana G (1998). DRB1*15 and DRB1*03 extended haplotype interaction in primary Sjögren's syndrome genetic susceptibility. Clin Exp Rheum 16:725–728.
Jimena P, Aguirre MA, Lopez-Curbelo A, de Andrès M, Garcia-Courtay C, and Cuadrado MJ (1998). Prolactin levels in patients with systemic lupus erythematosus: A case controlled study. Lupus 7:383–386.
Larrea F, Escorza A, Granados J, Valencia X, Valero A, Cravioto MC, and Perez-Palacios G (1987). Familial occurrence of big-big prolactin as the predominent immunoreactive human prolactin species in blood. Fertil Steril 47:956–963.
Larrea F, Marinez-Castillo A, Cabrera V, Alcocer-Varela J, Queipo G, Carino C, and Alarcon-Segovia D (1997). A bioactive 60-kilodalton prolactin species is preferentially secreted in cultures of mitogen-stimulated and nonstimulated peripheral blood mononuclear cells from subjects with systemic lupus erythematosus. J Clin Endocrinol Metab 82:3664–3669.
Mittra I (1980). A novel “cleaved prolactin” in the rat pituitary: I Biosynthesis, characterization and regulatory control. Biochem Biophys Res Commun 95:1750–1759.
Moutsopoulos HM (1994). Sjögren's syndrome: Autoimmune epithelitis. Clin Immunol Immunopathol 72:162–165.
Murphy LC, Murphy LJ, Tsuyushi D, Duckworth ML, and Shiu R (1988). Cloning and characterization of a cDNA encoding a highly conserved, putative calcium binding protein, identified by an antiprolactin receptor antiserum. J Biol Chem 263:2397–2401.
Neidhart M (1996). Elevated serum prolactin or elevated prolactin/cortisol ratio are associated with autoimmune processes in systemic lupus erythematosus and other connective tissue diseases. J Rheumatol 23:476–481.
Owerbach D, Rutter WJ, Cooke NE, Martial JA, and Shows TB (1981). The prolactin gene is located on chromosome 6 in humans. Science 212:815–816.
Polihronis M, Tapinos NI, Theocharis SE, Economou A, Kittas C, and Moutsopoulos HM (1998). Modes of epithelial cell death and repair in Sjögren's syndrome. Clin Exp Immunol 114:485–490.
Sabhrawal P, Glaser R, Lafuse W, Varma S, Liu Q, Arkins S, Kooijman R, Kutz L, Kelley KW, and Malarkey WB (1992). Prolactin synthesized and secreted by human peripheral blood mononuclear cells: An autocrine growth factor for lymphoproliferation. Proc Natl Acad Sci USA 89:7713–7716.
Salmon I, Camby I, Remmeling M, Rombaut K, Pasteels JL, Brotchi J, Kiss R, and Danguy R (1996). Lectin histochemistry, ploidy level and proliferation indices in meningioma subtypes. Neuropathol Appl Neurobiol 22:68–76.
Shoupe D, Montz FJ, Kletzy OA, and diZerega GS (1983). Prolactin molecular heterogeneity. Am J Obstet Gynecol 147:482–487.
Skopouli FN, Kousvelari EE, Mertz P, Jaffe ES, Fox PC, and Moutsopoulos HM (1992). c-myc mRNA expression in minor salivary glands of patients with Sjögren's syndrome. J Rheumatol 19:693–696.
Struman I, Bentzien F, Lee H, Mainfroid V, D'Angelo G, Goffin V, Weiner RI, and Martial JA (1999). Opposing actions of intact and N-terminal fragments of the human prolactin/growth hormone family members on angiogenesis: An efficient mechanism for the regulation of angiogenesis. Proc Natl Acad Sci USA 96:1246–1251.
Vitali C, Bombardieri S, Moutsopoulos HM, Balestrieri G, Bencivelli W, Bernstein RM, Bjerrum KB, Braga S, Coll J, de Vita S, Drosos AA, Ehrenfeld M, Harton PY, Hay EM, Isenberg DA, Janin A, Kalden JR, Kater L, Konttinen YT, Maddison PJ, Maini RN, Manthorpe R, Meyer O, Ostuni P, Pennec Y, Prause JU, Richards A, Sauvezie B, Schiødt M, Sciuto M, Scully C, Shoenfeld Y, Skopouli FN, Smolen JS, Snaith ML, Tishler M, Todesco S, Valesini G, Venables PJW, Wattiaux MJ, and Youinou P (1993). Preliminary criteria for the classification of Sjögren's syndrome: Results of a prospective concerted action supported by the European community. Arthritis Rheum 36:340–347.
Von Werder K and Clemm C (1974). Evidence for “big” and “little” components of circulating immunoreactive prolactin in humans. FEBS Lett 47:181–185.
Warhen M, Tengnèr P, Gunnarsson I, Lundberg I, Hedfors E, Ringertz NR, and Petterson I (1998). Ro/SS-A and La/SS-B antibody level variation in patients with Sjögren's syndrome and systemic lupus erythematosus. J Autoimmun 11:29–34.
Wu H, Devi R, and Malarkey WB (1996). Expression and localization of prolactin messenger ribonucleic acid in the human immune system. Endocrinology 137:349–353.
Acknowledgements
Granted by Fonds National de la Recherche Scientifique Médicale (FRSM and FNRS), Belgium, and the Swiss National Science Foundation (31–50510.97).
Author information
Authors and Affiliations
Corresponding author
Additional information
CD is a Research Associate and RK is a Senior Research Associate with the Fonds National de la Recherche Scientifique (FNRS), Belgium.
Rights and permissions
About this article
Cite this article
Steinfeld, S., Rommes, S., François, C. et al. Big Prolactin 60 kDa is Overexpressed in Salivary Glandular Epithelial Cells from Patients with Sjögren's Syndrome. Lab Invest 80, 239–247 (2000). https://doi.org/10.1038/labinvest.3780027
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.3780027
This article is cited by
-
Sex differences in Sjögren’s syndrome: a comprehensive review of immune mechanisms
Biology of Sex Differences (2015)
-
Prolactin and Autoimmunity
Clinical Reviews in Allergy & Immunology (2012)
-
Prolactin and Autoimmunity
Clinical Reviews in Allergy & Immunology (2011)
-
Prolactin Up-Regulates Cathepsin B and D Expression in Minor Salivary Glands of Patients with Sjögren's Syndrome
Laboratory Investigation (2000)