Abstract
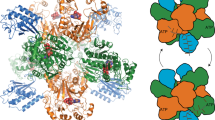
PENICILLIN antibiotics are all produced from fermentation-derived penicillins because their chemical synthesis is not commercially viable. The key step in penicillin biosynthesis, in which both the β-lactam and thiazolidine rings of the nucleus are created, is mediated by isopenicillin N synthase (IPNS), which binds ferrous iron and uses dioxygen as a cosubstrate. In a unique enzymatic step, with no chemical precedent, IPNS catalyses the transfer of four hydrogen atoms from its tripeptide substrate to dioxygen forming, in a single reaction, the complete bicyclic nucleus of the penicillins1. We now report the structure of IPNS complexed with manganese, which reveals the active site is unusually buried within a ဘjelly-rollမ motif and lined by hydrophobic residues, and suggest how this structure permits the process of penicillin formation. Sequence analyses indicate IPNS, 1-aminocyclopropane-l-carboxylic acid oxidase and many of the 2-oxo-acid-dependent oxygenases contain a conserved jelly-roll motif, forming a new structural family of enzymes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Pang, C.-P. et al. Biochem. J. 222, 789–795 (1984).
Fleming, A. Brit. J. exp. Path. 10, 226–236 (1929).
Florey, H. W. et al. Antibiotics Vol. 2 (Oxford Univ. Press, London, New York, 1949).
Crowfoot, D., Bunn, C. W., Rogers-Low, B. W. & Turner-Jones, A. The Chemistry of Penicillin (eds Clark, H. T., Johnson, J. R. & Robinson, R.) 310–321 (Princeton Univ. Press, Princeton, New Jersey, 1949).
Baldwin, J. E. & Schofield, C. J. in The Chemistry of ß-lactams (ed. Page, M. I.) Chapter 1 (Blackie, London, 1992).
Feig, A. L. & Lippard, S. J. Chem. Rev. 94, 759–805 (1994).
Fujishima, Y. et al. J. molec. Biol. 242, 712–714 (1994).
Baldwin, J. E., Blackburn, J. M., Sutherland, J. D. & Wright, M. C. Tetrahedron 47, 5991–6002 (1991).
Roach, P. L., Schofield, C. J., Baldwin, J. E., Clifton, I. J. & Hajdu, J. Prot. Sci. 4, 1007–1009 (1995).
Stuart, D. I. Curr. Opin. struct. Biol. 3, 167–174 (1993).
Norris, G. E., Sullivan, T. J., Anderson, B. F. & Baker, E. N. Structure 2, 1049–1059 (1994).
Srinivasan, N. et al. Structure 2, 1017–1027 (1994).
Retey, J. Angew. Chem. 29, 355–361 (1990).
Prescot, A. G. J. exp. Bot. 44, 849–861 (1993).
Marsh, E. N., Chang, M.D-T. & Townsend, C. A. Biochemistry 31, 12648–12657 (1992).
Barton, G. J. Meth. Enzym. 183, 403–428 (1990).
Barton, G. J. Prot. Engng 6, 37–4O (1993).
Baldwin, J. E. & Bradley, M. Chem. Rev. 90, 1079–1088 (1990).
Otwinowski, Z. in Data Collection and Processing (eds Sawyer, L., Isaacs, N. W. & Bailey, S.) 55–62 (DL/SCI/R34, Daresbury Laboratory, Warrington, U.K., 1993).
Cowtan, K. Acta crystallogr. D5O, 760–763 (1994).
Jones, T. A., Zou, J. Y., Cowan, S. W. & Kjeldgaard, M. Acta crystallogr. A47, 110–119 (1991).
Jones, T. A. & Thirup, S. EMBO J. 5, 819–822 (1986).
Brünger, A. T., Kuriyan, J. & Karplus, M. Science 235, 458–460 (1987).
Brünger, A. T. Nature 355, 472–474 (1992).
Read, R. J. Acta crystallogr. A42, 140–149 (1986).
Kraulis, P. J. J. appl. Crystallogr. 24, 946–950 (1991).
Orville, A. M. et al. Biochemistry 31, 4602–4612 (1992).
Randall, C. R. et al. Biochemistry 32, 6664–6673 (1993).
Scott, R. A., Wang, S., Eidsness, M. K., Kriauciunas, A. & Chen, V. J. Biochemistry 31, 4596–4601 (1992).
Chen, V. J. et al. J. biol. Chem. 264, 21677–21681 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Roach, P., Clifton, I., Fülöp, V. et al. Crystal structure of isopenicillin N synthase is the first from a new structural family of enzymes. Nature 375, 700–704 (1995). https://doi.org/10.1038/375700a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/375700a0
This article is cited by
-
Molecular insights into the unusually promiscuous and catalytically versatile Fe(II)/α-ketoglutarate-dependent oxygenase SptF
Nature Communications (2022)
-
Crystal structure and catalytic mechanism of the MbnBC holoenzyme required for methanobactin biosynthesis
Cell Research (2022)
-
Genome-wide identification, characterization and expression profiling of gibberellin metabolism genes in jute
BMC Plant Biology (2020)
-
Metabolic control of gene transcription in non-alcoholic fatty liver disease: the role of the epigenome
Clinical Epigenetics (2019)
-
Pro-metastatic collagen lysyl hydroxylase dimer assemblies stabilized by Fe2+-binding
Nature Communications (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.