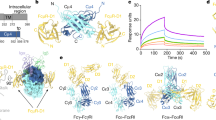
Abstract
The three-dimensional structure of the rat neonatal Fc receptor (FcRn) is similar to the structure of molecules of the major histocompatibility complex (MHC). The counterpart of the MHC peptide-binding site is closed in FcRn, making the FcRn groove incapable of binding peptides. A dimer of FcRn heterodimers seen in the crystals may represent a receptor dimer that forms when the Fc portion of a single immunoglobulin binds. An alternative use of the MHC fold for immune recognition is indicated by the FcRn and FcRn/Fc co-crystal structures.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Ravetch, J. V. Cell 78, 553–560 (1994).
Rodewald, R. J. Cell Biol. 45, 635–640 (1970).
Rodewald, R. J. Cell Biol. 71, 666–670 (1976).
Roberts, D. M., Guenthert, M. & Rodewald, R. J. Cell Biol. 111, 1867–1876 (1990).
Story, C. M., Mikulska, J. E. & Simister, N. E. J. exp. Med. 180, 2377–2381 (1994).
Simister, N. E. & Mostov, K. E. Nature 337, 184–187 (1989).
Ahouse, J. J. et al. J. Immun. 151, 6076–6088 (1993).
Simister, N. E. & Rees, A. R. Eur. J. Immun. 15, 733–738 (1985).
Townsend, A. & Bodmer, H. A. Rev. Immun. 7, 601–624 (1989).
Bjorkman, P. J. et al. Nature 329, 506–512 (1987).
Garrett, T. P. J., Saper, M. A., Bjorkman, P. J., Strominger, J. L. & Wiley, D. C. Nature 342, 692–696 (1989).
Madden, D. R., Gorga, J. C., Strominger, J. L. & Wiley, D. C. Cell 70, 1035–1048 (1992).
Fremont, D. H., Matsumura, M., Stura, E. A., Peterson, P. A. & Wilson, I. A. Science 257, 919–927 (1992).
Zhang, W. G., Young, A. C. M., Imarai, M., Nathenson, S. G. & Sacchettini, J. C. Proc. natn. Acad. Sci. U.S.A. 89, 8403–8407 (1992).
Young, A. C. M., Zhang, W., Sacchettini, J. C. & Nathenson, S. G. Cell 76, 39–50 (1994).
Gastinel, L. N., Simister, N. E. & Bjorkman, P. J. Proc. natn. Acad. Sci. U.S.A. 89, 638–642 (1992).
Burmeister, W. P., Huber, A. H. & Bjorkman, P. J. Nature 372, 379–383 (1994).
Jones, T. A. a, yaap, asap, (a #*? A Set of Averaging Programs (SERC, Daresbury, UK, 1992).
Brünger, A. T. X-PLOR (Version 3.1): A System for Crystallography and NMR (Yale University, New Haven, CT, 1992).
Hope, H. A. Rev. Biophys. biophys. Chem. 19, 107–126 (1990).
Brünger, A. T. Nature 355, 472–475 (1992).
Hodel, A., Kim, S.-H. & Brünger, A. T. Acta crystallogr. A48, 851–858 (1992).
Raghavan, M., Gastinel, L. N. & Bjorkman, P. J. Biochemistry 32, 8654–8660 (1993).
Brown, J. H. et al. Nature 364, 33–39 (1993).
Davies, D. R. & Metzger, H. A. Rev. Immun. 1, 87–117 (1983).
Lancet, D., Parham, P. & Strominger, J. L. Proc. natn. Acad. Sci. U.S.A. 76, 3844–3848 (1979).
Saper, M. A., Bjorkman, P. J. & Wiley, D. C. J. molec. Biol. 219, 277–319 (1991).
Stern, L. J. et al. Nature 368, 215–221 (1994).
Matsumura, M., Fremont, D. H., Peterson, P. A. & Wilson, I. A. Science 257, 927–934 (1992).
Kabat, E. A., Wu, T. T., Perry, H. M., Gottesman, K. S. & Foeller, C. Sequences of Proteins of Immunological Interest (US Department of Health and Human Services, Bethesda, MD, 1991).
Fahnestock, M. L., Tamir, I., Narhi, L. & Bjorkman, P. J. Science 258, 1658–1662 (1992).
Huber, A. H., Kelley, R. F., Gastinel, L. N. & Bjorkman, P. J. J. molec. Biol. 230, 1077–1083 (1993).
Beavil, A. J. et al. Biochem. Soc. Trans. 21, 968–972 (1993).
Jardetzky, T. S. et al. Nature 368, 711–718 (1994).
Raghavan, M., Chen, M. Y., Gastinel, L. N. & Bjorkman, P. J. Immunity 1, 303–315 (1994).
Salter, R. D. et al. Nature 345, 41–46 (1990).
Deisenhofer, J. Biochemistry 20, 2361–2370 (1981).
Leahy, D. J., Axel, R. & Hendrickson, W. A. Cell 68, 1145–1162 (1992).
Martin, L. H., Calabi, F., Lefevre, F.-A., Bilsland, C. A. & Milstein, C. Proc. natn. Acad. Sci. U.S.A. 84, 9189–9193 (1987).
Schall, S. & Barnard, E. A. J. molec. Biol. 41, 237–251 (1969).
Teng, T.-Y. J. appl. Crystallogr. 23, 387–391 (1990).
CCP4. The SERC (UK) Collaborative Computing Project No. 4, a Suite of Programs for Protein Crystallography (Daresbury Laboratory, Warrington WA4 4AD, UK, 1979).
Otwinowski, Z. in Isomorphous Replacement and Anomalous Scattering 80–86 (Daresbury Laboratory, UK, 1991).
Leslie, A. G. W. Acta crystallogr. A43, 134–136 (1987).
Jones, T. A., Bergdoll, M. & Kjeldgaard, M. Crystallographic Computing and Modeling Methods in Molecular Design (Springer, New York, 1993).
Brünger, A. T. Acta crystallogr. A46, 46–57 (1990).
Jones, T. A., Zou, J.-Y., Cowan, S. W. & Kjeldgaard, M. Acta crystallogr. A47, 110–119 (1991).
Kraulis, P. J. J. appl. Crystallogr. 24, 946–950 (1991).
Nicholls, A., Sharp, K. A. & Honig, B. Protein Struct. Funct. Genet. 11, 281–296 (1991).
Evans, S. V. J. molec. Graph. 4, 134–138 (1993).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Burmeister, W., Gastinel, L., Simister, N. et al. Crystal structure at 2.2 Å resolution of the MHC-related neonatal Fc receptor. Nature 372, 336–343 (1994). https://doi.org/10.1038/372336a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/372336a0
This article is cited by
-
The therapeutic age of the neonatal Fc receptor
Nature Reviews Immunology (2023)
-
Neonatal Fc Receptor–Targeted Therapies in Neurology
Neurotherapeutics (2022)
-
An intact C-terminal end of albumin is required for its long half-life in humans
Communications Biology (2020)
-
Reduced FcRn-mediated transcytosis of IgG2 due to a missing Glycine in its lower hinge
Scientific Reports (2019)
-
Human cytomegalovirus evades antibody-mediated immunity through endoplasmic reticulum-associated degradation of the FcRn receptor
Nature Communications (2019)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.