Abstract
Constitutive nuclear factor κB (NF-κB) activation characterizes Hodgkin/Reed-Sternberg (H-RS) cells. Blocking constitutive NF-κB has been shown to be a potential strategy to treat Hodgkin lymphoma (HL). Here, for the first time we show that although constitutive NF-κB level of H-RS cell lines is very high, topoisomerase inhibitors further enhance NF-κB activation through IκB kinase activation in not only H-RS cell lines with wild-type IκBα, but also in those with IκBα mutations and lacking wild-type IκBα. Thus, both constitutive and inducible NF-κB are potential targets to treat HL. We also present the data that indicate the involvement of IκBβ in NF-κB induction by topoisomerase inhibitors. A new NF-κB inhibitor, dehydroxymethylepoxyquinomicin (DHMEQ) inhibited constitutive NF-κB activity and induced apoptosis of H-RS cell lines. DHMEQ also inhibited the growth of H-RS cells without significant systemic toxicity in a NOD/SCID/γcnull (NOG) mice model. DHMEQ and topoisomerase inhibitors revealed enhancement of apoptosis of H-RS cells by blocking inducible NF-κB. Results of this study suggest that both constitutive and inducible NF-κB are molecular targets of DHMEQ in the treatment of HL. The results also indicate that IκBβ is involved in NF-κB activation in H-RS cells and IκBβ substitutes for IκBα in H-RS cells lacking wild-type IκBα.
Similar content being viewed by others
Main
Advances in chemotherapy and radiotherapy regimens for treating Hodgkin lymphoma (HL) represent a significant breakthrough in clinical oncology and have increased the long-term survival rate from 5% in the 1960s to greater than 80%. Today, problems of late side effects by chemotherapy such as secondary malignancies, myelodysplasia and cardio-toxicities and chemotherapy-resistant cases with poor prognosis have become important issues to still be resolved.1 Recently, a strategy that targets the molecules critical for maintenance and growth of the tumor cells has been thought to be a key to develop more effective treatment with less undesirable effects.2 This strategy intensifies the specificity of treatments to tumor cells and minimizes undesirable effects to normal cells. To establish a molecular target strategy for HL, it appears critical to identify the biological basis involved in anti-apoptosis of HL and develop specific agents that target this pathway.
It was found that constitutively activated nuclear factor-κB (NF-κB) is a molecular hallmark and survival mechanism for Hodgkin/Reed–Sternberg (H-RS) cells. Defective IκBα has been reported to be a cause of constitutive NF-κB activation in H-RS cells bearing IκBα gene mutations.3, 4, 5, 6, 7 We also reported that ligand-independent signals from overexpressed CD30 is responsible for constitutive NF-κB activation.8 Constitutively activated NF-κB is considered to be responsible for aberrant growth and cytokine gene expression of H-RS cells.9, 10 We reported that adenovirus-mediated gene transfer of a dominant IκBα resistant to phosphorylation-mediated degradation or a decoy CD30 lacking a tumor necrosis factor receptor associated factor (TRAF) binding domain can block the constitutive NF-κB activity and induce apoptosis in H-RS cell lines.8 These observations suggest that NF-κB, which is strongly and constitutively activated in H-RS cells, provides a molecular target for the treatment of HL.
Recently, NF-κB activation has also been connected with chemo-resistance of tumor cells.11 Topoisomerases are essential for cell survival and crucial for multiple aspects of DNA metabolism. Topoisomerases control the degree of supercoiled DNA, and whereas type I is crucial for transcription, type II is necessary for DNA replication. In both situations, the enzyme creates transient single-strand breaks in DNA, and the inhibitors prevent the replication of the strand breaks. SN-38, a powerful active metabolite of CPT-11, targets topoisomerase I activity, whereas daunorubicin and etoposide are topoisomerase II inhibitors. Treatments with topoisomerase inhibitors induce transient NF-κB activation via IκB kinase (IKK), which makes tumor cells resistant to induction of apoptosis.12
Previous reports suggested that blockade of the SN-38-induced activation of NF-κB by adenovirus-mediated gene transfer of IκBα super-repressor enhanced anti-tumor activity.13 Therefore, if topoisomerase inhibitors induce NF-κB activation in H-RS cells, inducible NF-κB also becomes a potential molecular target to treat HL. As tumor cells used in previous experiments did not show constitutive NF-κB activity, it remains to be examined whether the same strategy can be applied to tumor cells of HL with constitutive activation of NF-κB, especially for those with IκBα mutations.
IκBα mutations result in production of truncated, nonfunctional IκBα protein. Existence of IκBα mutations and lack of wild-type IκBα has been thought to make NF-κB activity uncontrolled by upstream IKK signals, leading to constitutive activation of NF-κB.3, 4, 5, 7 NF-κB induction by topoisomerase inhibitors has been reported to be mediated by IKK.12, 14 Therefore, it can be hypothesized that treatment with topoisomerase inhibitors will fail to induce NF-κB activity in H-RS cell lines with IκBα mutations.
Dehydroxymethylepoxyquinomicin (DHMEQ) is a new NF-κB inhibitor that is 5-dehydroxymethyl derivative of a novel compound epoxyquinomicin C having a 4-hydroxy-5,6-epoxycyclohexenone structure like panepoxydone.15 Panepoxydone had been found to inhibit TNF-α-induced activation of NF-κB. We have shown that DHMEQ inhibits NF-κB at the level of nuclear translocation.15
In the present study, we demonstrate that topoisomerase inhibitors induce NF-κB in not only H-RS cell lines with wild-type IκBα but also in H-RS cell lines lacking wild-type IκBα by activating IKK and constitutive as well as inducible NF-κB are the molecular target of HL treatment by DHMEQ. The results also indicate that NF-κB activity in H-RS cells with IκBα mutations is regulated by IκBβ, which is a substitute for IκBα.
Materials and methods
Cell Cultures
H-RS cell lines (KMH2, L428, HDLM2 and L540) were purchased from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). Cell lines were cultured in RPMI 1640 with supplementation of recommended concentrations of fetal bovine serum (FBS) and antibiotics. Peripheral blood mononuclear cells (PBMC) obtained from healthy volunteers were cultured in RPMI 1640 supplemented with 20% FBS and antibiotics.
Chemicals
DHMEQ is a NF-κB inhibitor that acts at the level of nuclear translocation of NF-κB.15, 16 Daunorubicin and etoposide were purchased from SIGMA (Toyko, Japan). SN-38, an active metabolite of camptothecin-11, was provided by Yakult (Tokyo, Japan). Compounds were dissolved with DMSO and used for experiments at the indicated concentrations. Bisbenzimide H 33342 fluorochrome (Hoechst 33342) was purchased from CALBIOCHEM (Bad Soden, Germany).
Electrophoretic Mobility Shift Analysis
Electrophoretic mobility shift analysis (EMSA) was carried out according to the methods described previously.17 Double-stranded oligonucleotide probes containing the mouse immunoglobulin kappa (Igκ) light-chain NF-κB consensus site and Oct-1 were purchased from Promega (Madison, WI, USA). Antibodies used for super-shift assays were as follows: NF-κB p50 (C-19) goat polyclonal antibody, RelB (C-19) rabbit polyclonal antibody and mouse antibodies for NF-κB p65 (C-20), NF-κB p52 (C-5) and c-Rel (B-6) (all from Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA).
In Vitro Kinase Assay
Cell extracts prepared from equivalent numbers of cells were subjected to immunoprecipitation with anti-IKKα monoclonal antibody in TNT buffer (20?mM TrisHCl (pH 7.5), 200?mM NaCl, 1% Triton X100, 0.5?mM PMSF, 1?mg/ml leupeptin, 1?mg/ml aprotinin, 100?μM Na3VO4 and 20?mM β-glycerophosphate). Immunoprecipitates were collected on Protein G-Sepharose beads (Amersham Biosciences Corp., Piscataway, NJ, USA), which were then washed three times with TNT buffer and three times with kinase reaction buffer (20?mM HEPES (pH 7.5), 10?mM MgCl2, 50?mM NaCl, 100?μM Na3VO4, 20?mM β-glycerophosphate, 2?mM DTT, and 20?mM ATP). Kinase reactions were performed for 30?min at 30°C using 5?μCi of [γ-32P]ATP and glutathione S-transferase (GST)-IκBα protein (amino acids 1317) (Santa Cruz Biotechnology, Inc.) as substrates. The reaction products were separated on 12% sodium dodecyl sulfate (SDS)–polyacrylamide gels and revealed by autoradiography. Antibodies for IKKα (B78-1) (BD Biosciences Pharmingen, San Diego, CA, USA) and IKKα/β (H-470) (Santa Cruz Biotechnology, Inc.) were used for immunoprecipitation and immunoblot of IKKα respectively.
Coimmunoprecipitation Analysis
Cell extracts were subjected to immunoprecipitation with anti-IκB-β (S-20) rabbit polyclonal antibody (Santa Cruz Biotechnology, Inc.) in TNE buffer (10?mM Tris–HCl, pH 7.8, 1% Nonidet P-40, 150?mM NaCl, 1?mM EDTA). Immunoprecipitates were collected on Protein G-Sepharose beads (Amersham Biosciences Corp.), which were then washed three times with TNE buffer.
Immunoblot Analysis
Immunoblot analysis was performed as described.17 Antibodies used were as follows: NF-κB p65 (F-6) mouse monoclonal antibody, NF-κB p50 (C-19) gout polyclonal antibody, IκB-β(S-20) rabbit polyclonal antibody, α tubulin (TU-02) mouse monoclonal IgM antibody (all from Santa Cruz Biotechnology, Inc.) and phosphoserine mouse monoclonal IgM antibody (Biomol, Meeting, PA, USA). Alkaline phosphatase-conjugated secondary antibodies are as follows: donkey anti-goat IgG antibody, donkey anti-mouse IgG antibody, donkey anti-rabbit IgG antibody (all from Chemicon International, Temecula, CA, USA) and goat anti-mouse IgM antibody (Santa Cruz Biotechnology, Inc.).
Cell Viability Assay
Effects of DHMEQ on cell viability were assayed by the MTT method as described previously.8 After incubation with DHMEQ or DMSO alone at the indicated concentrations and time points, 5 × 104 cells treated with MTT solution were measured by a microplate reader (Bio-Rad, Richmond, CA, USA) at a reference wavelength of 570?nm and test wavelength of 450?nm. The cell viability was expressed as a percentage of the DMSO-treated control samples.
Immunohistochemistry
Immunohistochemical analyses were carried out as described.17 Primary antibodies used were as follows: mouse monoclonal antibody for activated NF-κB p65 (Chemicon International), rabbit polyclonal antibodies for cleaved caspase-3 (Asp-175) (Cell signalling, Beverly, MA, USA), NF-κB p65 (C-20), Bcl-xL (H-62), FLIPs/l (H-202), GAPDH (FL-335) and mouse monoclonal antibody for α tubulin (TU-02) (all from Santa Cruz Biotechnology, Inc.). Fluorochrome-labeled secondary antibodies used in these studies are as follows: FITC-labeled anti-goat immunoglobulin donkey antibody, FITC-labeled anti-rabbit immunoglobulin goat antibody and FITC-labeled anti-mouse immunoglobulin goat antibody (all from Santa Cruz Biotechnology, Inc.).
Northern Blotting
Northern blot analysis was carried out essentially as described.17 Briefly, 2?μg of poly(A)-selected RNA was sizefractionated on 1% formalin agarose gel electrophoresis and subsequently blotted onto Hybond-C extra-nitrocellulose membranes (Amersham Bioscience Corp.). Filters were hybridized in 4 × SSC, 1 × Denhardts, 0.5% SDS, 0.1?M NaPO4 (pH 7.0), 10% Dextran-Na at 65°C with 1.0 × 106?cpm/ml of random prime-labeled probes. After washings to a final stringency of 0.2 × SSC and 0.1% SDS at 65°C, filters were exposed to XAR-5 films (Eastman Kodak, Rochester, NY, USA) at −80°C. RT-PCR amplified cDNA fragments of human Bcl-xL, human FLIPs/l and human GAPDH were used as probes.
Apoptosis and Analysis of Caspase Activity
Cells were labeled with FITC-conjugated Annexin V (BD Biosciences, Pharmingen) and followed by flow cytometric analysis. For analysis of morphological changes of nuclei, cells were stained by 10?μM Hoechst 33342, and photographed through a UV filter. Activation of caspases was examined by detecting decrease of uncleaved fragments or appearance of cleaved fragments with immunoblot analysis. Antibodies used in these experiments were as follows: mouse monoclonal antibodies for caspase-3/CPP32 (BD Biosciences Pharmingen), cleaved caspase-8 (Asp384) 11G10 and rabbit polyclonal antibodies for caspase-9 (both from Cell Signaling). Alkaline phosphatase-conjugated secondary antibodies are as follows: anti-mouse IgG (H&L) antibody and anti-rabbit IgG (Fc) antibody (both from Promega). caspase activity was blocked using cell-permeable irreversible caspase-3 inhibitor II (z-DEVD-FMK), caspase 8 inhibitor II (z-IETD-FMK) or caspase-9 inhibitor I (z-LEHD-FMK) (all from CALBIOCHEM).
In Vivo Therapeutic Effect of DHMEQ
NOG mice were purchased from the Central Institute for Experimental Animals (Kawasaki, Japan). The Ethical Review Committee of the National Institute of Infectious Diseases approved the experimental protocol. H-RS cells were inoculated subcutaneously into the post-auricular area of NOG mice. DHMEQ was administered intraperitoneally three times a week for 1 month to mice at doses of 12?mg/kg, beginning on either day 0 or day 5 when tumors were palpable. The control mice were inoculated RPMI 1640 (200?μl). Mice were sacrificed at 1-month follow-up period after inoculation. Tumor tissues were fixed with Streck Tissue Fixative (STF) and processed to paraffin wax-embedded sections for staining with hematoxylin and eosin (HE), as described.18
Statistical Analysis
Differences between mean values were assessed by two-tailed t-test. A P-value <0.05 was considered to be statistically significant.
Results
Topoisomerase Inhibitors Induce Transient NF-κB Activation in H-RS Cell Lines Independent of IκBα Mutations
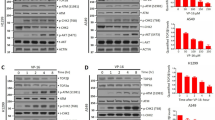
We first examined effects of topoisomerase inhibitors on the levels of NF-κB activity in H-RS cell lines without IκBα mutation (L540 and HDLM2) as well as in H-RS cells with IκBα mutations and lacking wild-type IκBα (KMH2 and L428). Treatment by SN-38, daunorubicin and etoposide further induced transient NF-κB activation, which peaked at 1 or 2?h after treatment in L540 without IκBα mutations (Figure 1a top). HDLM2, which also lacks IκBα mutation, showed a similar result (data not shown). Surprisingly, NF-κB induction, which peaked later at 4 or 5?h, was observed in H-RS cell line KMH2 bearing IκBα mutations (Figure 1a bottom). L428 bearing IκBα mutations showed transient NF-κB activation, which peaked at 1?h after treatment (data not shown). Supershift analyses using nuclear extracts prepared from H-RS cell lines at the time point of maximal NF-κB induction revealed that NF-κB includes p50 and p65. The representative results of KMH2 cells are shown in Figure 1b. Next we examined whether NF-κB induction by topoisomerase inhibitors in H-RS cell lines is mediated by IKK activation. In vitro kinase assay clearly showed transient activation of IKK after SN-38 treatment in both L540 and KMH2 cells (Figure 1c). These results indicate that transient NF-κB induction, which is induced independent of IκBα mutations by topoisomerase inhibitors is mediated by activation of the IKK pathway and not only constitutive, but also inducible NF-κB is a potential molecular target for treatment of HL.
Treatment by topoisomerase inhibitors further induces NF-κB activity via IKK in H-RS cell lines with or without IκBα mutations. (a) The effect of SN-38, daunorubicin and etoposide on NF-κB activity of H-RS cells with or without IκBα mutations. H-RS cell lines; L540 cells without IκBα mutations or KMH2 cells with IκBα mutations were treated with 100?ng/ml of SN-38, 2?μM daunorubicin or 50?μM etoposide for indicated hours. Nuclear extracts (1?μg) were examined for NF-κB-binding activity by EMSA with radiolabeled NF-κB specific probe. Topoisomerase inhibitors used are indicated above. DNR, daunorubicin; ETP, etoposide. Lower panels show results of EMSA with a control probe, Oct-1. (b) Analysis of NF-κB subcomponent after treatment with topoisomerase inhibitors by supershift assay. KMH2 cells were treated with topoisomerase inhibitors for 5?h and harvested.Two micrograms of nuclear extracts were subjected to analysis. Antibodies used are indicated above. (c) In vitro kinase assay of H-RS cell lines with or without IκBα mutations. L540 cell or KMH2 cells treated with 100?ng/ml of SN-38 for indicated hours were immunoprecipitated by anti-IKK antibody and subjected to in vitro kinase assay using IκBα as substrate. Phosphorylation of IκBα by IKK, which represents IKK activity, is shown in the upper panels. Immunoblot of immunoprecipitates by anti-IKK antibody in lower panels shows an equal amount of IKK was used in each reaction.
Involvement of IκBβ in the Topoisomerase Inhibitor-Mediated Induction of NF-κB in H-RS Cells with IκBα Mutations
Previous reports indicated the functional redundancy and similar kinetics of IκBα and IκBβ.19 Therefore, we examined the involvement of IκBβ in the topoisomerase inhibitor-mediated induction of NF-κB in H-RS cells with IκBα mutations and lacking IκBα. To address this point, we examined the binding of p65 and p50 with IκBβ, and the degradation and phosphorylation of IκBβ after SN-38 treatment. Previous reports indicated that like IκBα, IκBβ is also regulated by the phosphorylation of serine residues near the N terminus.20 We used anti-phosphoserine antibody to detect phosphorylation of IκBβ. We also examined by confocal microscopy the distribution of activated NF-κB p65 and IκBβ after SN-38 treatment.
Both p65 and p50 were immunoprecipitated along with IκBβ in L428 cells (Figure 2a). In L428 cells, decreased expression of IκBβ was observed from 0.5 to 1?h after SN-38 treatment (Figure 2b). The decreased expression of IκBβ was preceded by phosphorylation of IκBβ (Figure 2c). Stimulation of L428 with SN-38 induced NF-κB activity, which peaked at 1?h (Figure 2d). Analysis by confocal microscopy revealed that active p65 NF-κB, which showed diffuse distribution before treatment, was concentrated in the nucleus 1?h after SN-38 treatment and was redistributed to the cytoplasm 2?h after SN-38 treatment (Figure 2e, top panels). IκBβ decreased the expression 1?h after SN-38 treatment (Figure 2e, bottom panels). These results indicate that IκBβ is involved in the topoisomerase inhibitor-mediated induction of NF-κB in H-RS cells lacking wild-type IκBα.
Topoisomerase inhibitor-mediated induction of NF-κB is mediated by the phosphorylation and degradation of IκBβ in H-RS cells with IκBα mutations. (a) Coimmuniprecipitation analysis of NF-κB p50 and p65 with IκBβ. Immunoprecipitates of anti-IκBβ antibody were blotted with anti-NF-κB p50 or p65 antibodies (upper panels). Immunoprecipitates were blotted with anti-IκBβ antibody (bottom panel). IP, immunoprecipitation. (b) Expression level of IκBβ protein in L428 cells after treatment with topoisomerase inhibitor. Whole cell lysates of L428 treated with SN-38 for the indicated number of hours were blotted with anti-IκBβ antibody (upper panel) or anti-α tubulin antibody (bottom panel). (c) Phosphorylation of IκBβ protein in L428 cells after treatment with topoisomerase inhibitor. L428 cells were treated with 100?ng/ml of SN-38 for the indicated number of hours. Immunoprecipitates of anti-IκBβ antibody were blotted with anti-phosphoserine antibody (upper panel) or anti-IκBβ antibody (bottom panel). (d) The effect of SN-38 on NF-κB activity in L428 cells. L428 cells were treated with 100?ng/ml of SN-38 for the indicated number of hours. Nuclear extracts (1?μg) were examined for NF-κB-binding activity by EMSA with radiolabeled NF-κB-specific probe. (e) Localization of activated NF-κB p65 and IκBβ after treatment with topoisomerase inhibitor. L428 cells were treated with 100?ng/ml of SN-38 for the indicated number of hours. Confocal immunofluorescence microscopic analysis was performed on cytospin samples stained with antibodies against activated NF-κB p65 and IκBβ.
DHMEQ Reduces Cell Growth and Induces Apoptosis of H-RS Cells Through Inhibition of Constitutive NF-κB Activity
We next examined the effects of DHMEQ on constitutive NF-κB activity in H-RS cell lines. Treatment with DHMEQ at a concentration of 10?μg/ml abrogated constitutive NF-κB-binding activity in these cell lines (Figure 3a). Inhibition of constitutive NF-κB activity by DHMEQ was observed in all H-RS cell lines examined irrespective of presence (L428 and KMH2) or absence (L540 and HDLM2) of IκBα mutations. Analysis by confocal microscopy revealed accumulation of active form of NF-κB p65 in the cytoplasm after DHMEQ treatment in KMH2 and L540, indicating action of DHMEQ at the level of translocation of NF-κB into the nucleus (Figure 3b). Time-course studies showed that DHMEQ almost completely abrogated NF-κB binding activity at 1 hour after DHMEQ treatment and thereafter for 16?h (Figure 3c). The supershift assays revealed that the affected NF-κB components include p50 and p65 as reported previously9 (Figure 3d).
DHMEQ suppresses constitutive NF-κB activity in H-RS cell lines. (a) Inhibition of constitutive NF-κB-binding activity in H-RS cell lines. H-RS cell lines, L428, KMH2, L540 and HDLM2, were treated with (+) or without (−) 10?μg/ml of DHMEQ for 7?h. Nuclear extracts (2?μg) were examined for NF-κB binding activity by EMSA with a radiolabeled NF-κB specific probe. The upper panel shows inhibition of NF-κB-binding activity by DHMEQ. The lower panel shows results of EMSA with a control probe, Oct-1. The position of shifted bands corresponding to NF-κB and free probes are indicated on the left. (b) Accumulation of active NF-κB p65 in the cytoplasm after DHMEQ treatment. KMH2 and L540 cells were treated with or without 10?μg/ml of DHMEQ for 6?h. Confocal immunofluorescence microscopic analysis was carried out on cytospin samples stained with antibody against active NF-κB p65. (c) Time-course studies of NF-κB inhibition by DHMEQ. KMH2 and L540 cells were treated with 10?μg/ml of DHMEQ for indicated hours. Nuclear extracts (2?μg) were examined for NF-κB-binding activity by EMSA with a radiolabeled NF-κB-specific probe. EMSA with Oct-1 served as control. (d) NF-κB subcomponent analysis in H-RS cell lines. Subcomponents of NF-κB constitutively activated in H-RS cell lines were determined by supershift analysis. Nuclear extracts (2?μg) of untreated H-RS cell lines were subjected to supershift analysis with antibodies specific for c-Rel, NF-κB p50 and NF-κB p65. Cell lines used are indicated on the left.
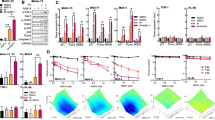
We next examined the effect of DHMEQ on viability of H-RS cell lines (Figure 4). Results of MTT assays showed that DHMEQ treatment reduced cell viability of all four H-RS-derived cell lines in a dose-dependent manner, whereas it did not show a significant effect on the viability of PBMC even at higher concentrations (Figure 4a). Furthermore, we examined whether DHMEQ induces apoptosis of H-RS cell lines by analyzing Annexin V reactivity and nuclear fragmentation. Based on the results obtained from MTT assays, L428 and KMH2 cells were treated with 20?μg/ml, and L540 and HDLM2 cells were treated with 10?μg/ml of DHMEQ. Flow cytometric analysis showed a significant increase in the number of Annexin V-positive cells after DHMEQ treatment in H-RS cell lines, but not in PBMC (Figure 4b). Hoechst 33342 staining showed fragmentation and condensation of the nucleus of H-RS cell lines, suggesting induction of apoptosis in these cells, but not in normal PBMC (Figure 4c).
DHMEQ induces apoptosis of H-RS cell lines. (a) Dose-dependent reduction of cell viabilities of H-RS cell lines treated with DHMEQ. H-RS cell lines; L428, KMH2, L540 and HDLM2 as well as PBMC were treated with indicated concentrations of DHMEQ for 48?h. Cell viabilities were determined by MTT assay. Data represent the mean±s.d. of three independent experiments. (b) Flow cytometric analysis of Annexin V-reactive cells. L428 and KMH2 cells were treated with 20?μg/ml of DHMEQ for indicated hours. L540 and HDLM2 cells were treated with 10?μg/ml of DHMEQ. PBMC were treated with 20?μg/ml of DHMEQ. After labeling with FITC-conjugated Annexin V, cells were analyzed by flow cytometry. Data represent the mean±s.d. of three independent experiments. (c) Nuclear fragmentation of cells treated with DHMEQ. Cells were treated with the same concentration of DHMEQ used in the detection of Annexin V reactive cells for 48?h and stained with 10?μM Hoechst 33342. Cells used are indicated above.
Taken together, these results indicate that DHMEQ selectively targets constitutive NF-κB activity in H-RS cells and induces apoptosis of these cells independent of IκBα mutations.
DHMEQ-Induced Apoptosis Involves Activation of Caspases 3, 8 and 9
To examine whether induction of apoptosis upon inhibition of constitutive NF-κB activity in H-RS cells by DHMEQ is caused by the activation of the caspase pathway, we first studied activation of caspase 3 by immunoblot analysis. Results clearly showed cleavage of caspase 3, suggesting DHMEQ-induced apoptosis is associated with activation of the caspase pathway (Figure 5a, top panel). To differentiate the membranous and mitochondrial pathways, we next examined activation of caspase 8 and 9 that are upstream of caspase 3 by immunoblot analysis. DHMEQ-treated H-RS cells showed activation of both caspases 8 and 9 (Figure 5a second and third panels). To confirm this, we next studied whether inhibitors of caspase 3, 8 and 9 can inhibit DHMEQ-induced apoptosis. The results demonstrated significant alleviation of apoptosis in the cells treated by these inhibitors, although inhibition of apoptosis was not complete (Figure 5b). These results suggested that the apoptosis induced by DHMEQ is mediated by both membranous and mitochondrial caspase pathways.
DHMEQ-induced apoptosis of H-RS cell lines involves activation of membranous and mitochondrial caspase pathways and downregulation of c-FLIP and Bcl-xL. (a) Immunoblot analyses of caspase -3, -8 and -9. L540 cells were treated with 10?μg/ml of DHMEQ and KMH2 cells were treated of 20?μg/ml with DHMEQ for indicated hours. 30?μg of whole cell lysates were subjected to the analysis. (b) Inhibition of DHMEQ induced apoptosis by blockade of caspase-3, -8 and -9 activities in KMH2 cells. Before the incubation with 15?μg/ml of DHMEQ, KMH2 cells were treated with 50?μM of caspase 3 inhibitor z-DEVD-FMK, caspase 8 inhibitor z-IETD-FMK or caspase 9 inhibitor z-LEHD-FMK. After 12?h of treatment with DHMEQ, cells were analyzed by MTT assay. α-Csp, anti-caspase. (c) The expression of Bcl-xL and c-FLIP mRNA. L428 and L540 cells were treated with or without 10?μg/ml of DHMEQ for 16?h. The expression of Bcl-xL and c-FLIP was examined by Northern blot analysis, using RT-PCR amplified fragments as probes. Two microgramof poly (A)-selected RNA were subjected to the analysis. Results of Northern blot analyses are shown on the left. Expression of GAPDH served as a control. Quantification of relative levels of expression is shown on the right. GAPDH signals were measured by densitometry and the values were used to normalize the levels of densitometric quantification of Bcl-xL and c-FLIP mRNA expression in L428 and L540 cells. The relative expression levels of treated samples are expressed as percentages of those of untreated ones, which are set to 100%. (d) Expression of Bcl-xL and c-FLIP proteins involved in anti-apoptosis. L428 and L540 cells were treated with or without 10?μg/ml of DHMEQ for 16?h. Cells were spun by centrifugation onto glass coverslips and stained with antibodies specific for Bcl-xL and c-FLIP and observed with fluorescence confocal microscopy. Expression of α-tubulin served as control.
Recent reports indicate frequent expression of anti-apoptotic genes Bcl-xL and c-FLIP whose constitutive induction are critically involved in anti-apoptotic activity in H-RS cells.21, 22, 23, 24 Bcl-xL and c-FLIP antagonize mitochondrial and membranous caspase activities.25 We next examined changes in their expression upon DHMEQ treatment by Northern blotting and immunohistochemistry. The result confirmed downregulation of Bcl-xL and c-FLIP mRNAs (Figure 5c). We also examined the protein expression of Bcl-xL and c-FLIP by immunofluorescence confocal microscopy. The results clearly showed downregulation of expression of these proteins (Figure 5d). Taken together, these data confirmed that DHMEQ induced apoptosis of H-RS cell lines is mediated via mitochondrial and membranous pathway, which are accompanied by downregulation of Bcl-xL and c-FLIP.
DHMEQ Shows a Potent Inhibitory Effect on the Growth of H-RS Cells in NOG Mice Model
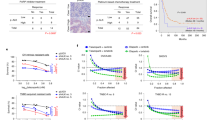
As the above results suggested potential efficacy of DHMEQ for the treatment of patients with HL by inhibiting constitutive NF-κB activity, we next examined whether DHMEQ treatment can suppress growth of xenografted H-RS cells in the NOG mice model. As expected, DHMEQ treatment resulted in reduction of the tumor mass at 1 month after inoculation (Figure 6a). A significant decrease in the size and weight of tumors in mice treated with DHMEQ was demonstrated when compared with controls at 1 month (Figure 6b). DHMEQ also inhibited the size and growth of tumors established by inoculation of L540 and KMH2 cells, indicating that the action of DHMEQ is independent of lack of wild-type IκBα as is expected by experiments in vitro (Figure 6c and d). DHMEQ at this treatment dosage (12?mg/kg of DHMEQ, three times a week for 1 month) is well tolerated without adverse findings such as weight loss or cachexia of treated mice. As expected, microscopic analysis of tumors revealed apoptotic cells in specimens from DHMEQ-treated mice (Figure 6e). These results suggest that DHMEQ contributes to the reduction of HL tumors independent of lack of IκBα in mice model.
Effects of DHMEQ on H-RS cell lines inoculated in NOG mice. A total of 1 × 107 cells were inoculated in the post-auricular region of NOG mice. For the treatment group, 12?mg/kg of DHMEQ was administered intraperitoneally three times a week for 1 month, beginning on either day 0 or day 5 when tumors were palpable and established. The control mice received RPMI 1640 (200?μl) simultaneously. (a) Gross appearance of the mice with (right) or without (left) DHMEQ treatment. Macroscopic images of subcutaneous tumors formed by L540 and those resected from mice with (right) or without (left) DHMEQ treatment, which began on day 0. (b) Size and weight of the resected tumors were measured and represented as bar graphs. Data represent the mean±s.d. from six mice. (c and d) Effects of DHMEQ on established tumors. L540 cells (c) and KMH2 cells (d) were used for the experiments. Size of tumors was measured and represented as bar graphs. Data represent the mean±s.d. from four mice. (e) Growth inhibitory effect of DHMEQ on L540 cells is accompanied by apoptosis. Microscopic images of HE-stained tumor tissues of mice with or without DHMEQ treatment (right and left, respectively) revealed apoptotic cells in DHMEQ-treated mice.
DHMEQ Enhances Anti-Tumor Effect of Topoisomerase Inhibitors by Blocking Inducible NF-κB in H-RS Cells
We next examined the effects of DHMEQ on NF-κB activity induced by topoisomerase inhibitors in KMH2 cells. Treatment by DHMEQ almost completely abrogated both constitutive and inducible NF-κB activities (Figure 7a). Analysis by confocal microscopy revealed accumulation of active form of NF-κB p65 in the cytoplasm of KMH2 cells treated with SN-38 and DHMEQ, supporting the notion that DHMEQ inhibits these NF-κB at the level of translocation into the nucleus (Figure 7b).
DHMEQ abrogates inducible NF-κB and enhances anti-tumor effect of topoisomerase inhibitors in H-RS cell lines. (a) Inhibition of topoisomerase inhibitors-mediated NF-κB induction by DHMEQ. KMH2 cells were exposed to 100?ng/ml of SN-38, 2?μM daunorubicin or 50?μM etoposide in combination with 10?μg/ml of DHMEQ for indicated hours. Two microgram of nuclear extracts were examined for NF-κB-binding activity by EMSA using NF-κB probe. Lower panels show results of EMSA with a control probe, Oct-1. DNR, daunorubicin; ETP, etoposide. (b) Accumulation of active NF-κB p65 after DHMEQ treatment. KMH2 cells were exposed to 100?ng/ml of SN-38 in combination with 10?μg/ml of DHMEQ for indicated hours. Confocal immunofluorescence microscopic analysis was carried out on cytospin samples stained with antibody against active NF-κB p65. (c) Effect of DHMEQ on viability of H-RS cells treated by topoisomerase inhibitors. KMH2 cells were treated with indicated concentrations of topoisomerase inhibitors with or without 10?μg/ml of DHMEQ. Forty-eight hours after treatment, cell viability was measured by MTT assay and the relative viability was determined. MTT values of DMSO-treated cells were set to 100%. Data present the mean±s.d. of three independent experiments. *P<0.05, compared with SN-38, DNR or ETP alone. (d) Analysis of Annexin V-reactive cells. KMH2 cells were treated with 10?μg/ml of DHMEQ with or without topoisomerase inhibitors for 24?h. Cells were stained by FITC-conjugated Annexin V and analyzed by flow cytometry. Data present the mean±s.d. of three independent experiments. Concentration of the agents was SN-38; 100?ng/ml, daunorubicine; 2?μM and etoposide; 50?μM. *P<0.05, compared with SN-38, DNR or ETP alone. (e) Nuclear fragmentation. KMH2 cells were treated with topoisomerase inhibitors with or without 10?μg/ml of DHMEQ for 24?h. After treatment, KMH2 cells were harvested and subjected to staining by Hoechst 33342. Concentration of the agents was the same as in flow cytometric analysis of Annexin V-reactive cells DHM, DHMEQ. (f) Activation of caspase-3. L428 cells were treated with 100?ng/ml of SN-38 with or without 10?μg/ml of DHMEQ for 24?h. Cells were spun onto slide glass and stained with antibody for cleaved caspase-3 and analyzed by confocal microscopy. Staining by GAPDH served as a control.
We next examined whether topoisomerase inhibitors and DHMEQ show enhanced anti-tumor effects in H-RS cell lines. We incubated KMH2 cells with sublethal concentrations of SN-38 with or without 10?μg/ml of DHMEQ for 48?h. The viability of the cells was measured by MTT assay. Combination of DHMEQ and SN-38 showed enhanced effect in the reduction of cell viability of KMH2 cells (Figure 7c left panel). Other topoisomerase inhibitors, daunorubicin and etoposide also revealed almost the same effects (Figure 7c middle and right panel).
To explore whether the combined effects result in enhanced induction of apoptosis, we examined expression of Annexin V, a marker for the early stage of apoptosis, and nuclear fragmentation of KMH2 cells. In each combination with one of three topoisomerase inhibitors, DHMEQ treatment enhanced Annexin V staining (Figure 7d) and fragmentation or condensation of the nuclei (Figure 7e). DHMEQ also enhanced SN-38-induced activation of caspase 3 in L428 cells (Figure 7f). These observations indicate that blockade of inducible NF-κB by DHMEQ enhances the anti-tumor effects of topoisomerase inhibitors in H-RS cells.
Discussion
In this study, we showed that although the NF-κB level of H-RS cell is very high, topoisomerase inhibitors further stimulated NF-κB activity through IKK activation in not only H-RS cell lines with wild-type IκBα, but also H-RS cell lines with defective IκBα. We presented the supportive evidence that IκBβ is involved in NF-κB induction in H-RS cells. We also showed that a new NF-κB inhibitor, DHMEQ-enhanced cytotoxicity of topoisomerase inhibitors by inhibiting inducible NF-κB, independent of the presence or absence of IκBα mutations in H-RS cell lines. The results suggest that constitutive and inducible NF-κB are appropriate molecular targets for the treatment of HL, and DHMEQ is a suitable compound to target these NF-κB.
Activation of IKK and IκBα upon NF-κB induction by topoisomerase inhibitors has been well-documented.12, 14 Topoisomerase inhibitors are thought to mobilize a preexisting signaling pathway that starts from the nucleus at the level of DNA strand breaks to end up in the cytosol at the IKK complex. Several candidate molecules such as ATM and DNA-PK involved in this step have been reported.26, 27 A previous study excluded involvement of an autocrine NF-κB activator synthesized by stimulation of topoisomerase inhibitors.12 Transient activation of IKK and NF-κB by topoisomerase inhibitors in H-RS cell lines indicates that similar kind of activation pathway also operates in H-RS cells having strong and constitutive NF-κB activity.
About 10–20% of H-RS cells are reported to harbor hetero- or homo-genetic alteration of IκBα genes resulting in the production of defective IκBα lacking C-terminal domain unable to bind with NF-κB. Lack of wild-type IκBα by IκBα mutations has been reported to one of the causes of constitutive NF-κB activation in H-RS cells.3, 4, 5, 6, 7 If IκBα is the only molecule that regulates NF-κB in H-RS cells, lack of wild-type IκBα may cause deregulated activation of NF-κB independent of upstream IKK signals. However, IKK-mediated induction of NF-κB by topoisomerase inhibitors in H-RS cell lines lacking wild-type IκBα suggests the existence of other molecules that regulate NF-κB activity by substitution for IκBα. Our previous result that adenovirus-mediated transduction of decoy CD30 lacking the cytoplasmic domain inhibits NF-κB activity and the recent report that proteasome inhibitor PS341 induces apoptosis in H-RS cell lines lacking wild-type IκBα, support the above hypothesis.8, 28 The results in this study indicate that IκBβ is involved in NF-κB activation in H-RS cells and IκBβ substitutes for IκBα in H-RS cells lacking wild-type IκBα. Functional redundancy and similar kinetics of activities of IκBα and IκBβ reported previously also support the above notion.19
The results obtained in this study suggest that not only constitutive, but also inducible NF-κB activities are good molecular targets of HL treatment. The results confirmed that topoisomerase inhibitors, SN-38, daunorubicin and etoposide can further induce transient NF-κB activation in addition to the basal strong and constitutive NF-κB activity in H-RS cell lines. Previous studies showed that stimulation of H-RS cell lines by TNF family members; CD40L or TNF cannot enhance NF-κB activity.5, 6 These observations indicate that in H-RS cells, signals from TNF receptor family members to IKK complex, which are mediated by TRAF proteins, are fully active, whereas IKK complex is still responsible for TRAF-independent stimulation. The demonstration that DHMEQ enhanced effects of topoisomerase inhibitors by blocking inducible NF-κB, suggests that inducible NF-κB blunts the effects of topoisomerase inhibitors in H-RS cells and DHMEQ can restore this effect.
Blockade of NF-κB is evident at 1?h and thereafter (Figure 3c), apoptosis induction was at a relatively late event after DHMEQ treatment (Figure 4b). Therefore, induction of apoptosis in H-RS cells by DHMEQ may be indirect and mediated by altered levels of gene expression. As inhibition of constitutive NF-κB activation is sufficient to trigger apoptosis of H-RS cells without stimulation of death receptor or cytotoxic agents, survival of H-RS cells appears to depend on a balance between anti-apoptotic and pro-apoptotic activities. In support of this notion, the present study confirmed the downregulation of c-FLIP and Bcl-xL, which are reported to be frequently expressed in H-RS cells upon blocking of NF-κB activity21, 22, 23, 24 (Figure 5c and d).
Prompt and specific action of DHMEQ suggests that DHMEQ is a suitable compound to target NF-κB in H-RS cells. Recent studies in other laboratories using gliotoxin, MG132, arcenic and PS341 also suggest that low-molecular-weight compounds have a potential to inhibit NF-κB in H-RS cells. However, their specificity for the NF-κB pathway appears to be relatively low when compared with that of DHMEQ.28, 29, 30 The target of DHMEQ resides downstream of targets of gliotoxin, MG132, arcenic and PS341. Furthermore, inhibition of NF-κB by gliotoxin, MG132, and PS341 is only one of the results of their activities as proteasome inhibitors.28, 29 Arsenic also alters a variety of enzymatic activities because of reactivity with sulfhydryl groups.30
The unique properties of DHMEQ appear to minimize adverse effects on normal cells. Notably, PBMC are resistant to apoptosis by DHMEQ treatment, although the mechanism is not currently understood. Results of our in vivo model suggest that DHMEQ may be minimally less toxic at effective doses. Treatment of mice with DHMEQ three times a week showed significant anti-tumor activity. DHMEQ treatment did not show significant systemic toxicity such as body weight loss in these experiments. The dose of DHMEQ used in these experiments (12?mg/kg) is far less than LD50 of DHMEQ that is 180?mg/kg (unpublished observation). Therefore, DHMEQ may be more suitable for NF-κB inhibition in H-RS cells.
In conclusion, both constitutive and inducible NF-κB are potential molecular targets to treat HL independent of the presence of IκBα mutations. NF-κB inhibitor DHMEQ is a suitable candidate to translate this strategy into clinical medicine. The results also indicate that IκBβ is involved in NF-κB activation in H-RS cells and IκBβ substitutes for IκBα in H-RS cells lacking wild-type IκBα.
References
Diehl V, Thomas RK, Re D . Part II: Hodgkin's lymphoma—diagnosis and treatment. Lancet Oncol 2004;5:19–26.
Griffin J . The biology of signal transduction inhibition: basic science to novel therapies. Semin Oncol 2001;28:3–8.
Emmerich F, Meiser M, Hummel M, et al. Overexpression of I kappa B alpha without inhibition of NF-kappaB activity and mutations in the I kappa B alpha gene in Reed-Sternberg cells. Blood 1999;94:3129–3134.
Jungnickel B, Staratschek-Jox A, Brauninger A, et al. Clonal deleterious mutations in the IkappaBalpha gene in the malignant cells in Hodgkin's lymphoma. J Exp Med 2000;191:395–402.
Krappmann D, Emmerich F, Kordes U, et al. Molecular mechanisms of constitutive NF-kappaB/Rel activation in Hodgkin/Reed-Sternberg cells. Oncogene 1999;18:943–953.
Wood KM, Roff M, Hay RT . Defective IkappaBalpha in Hodgkin cell lines with constitutively active NF-kappaB. Oncogene 1998;16:2131–2139.
Cabannes E, Khan G, Aillet F, et al. Mutations in the IkBa gene in Hodgkin's disease suggest a tumour suppressor role for IkappaBalpha. Oncogene 1999;18:3063–3070.
Horie R, Watanabe T, Morishita Y, et al. Ligand-independent signaling by overexpressed CD30 drives NF-kappaB activation in Hodgkin/Reed-Sternberg cells. Oncogene 2002;21:2493–2503.
Bargou RC, Leng C, Krappmann D, et al. High-level nuclear NF-kappa B and Oct-2 is a common feature of cultured Hodgkin/Reed-Sternberg cells. Blood 1996;87:4340–4347.
Bargou RC, Emmerich F, Krappmann D, et al. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin's disease tumor cells. J Clin Invest 1997;100:2961–2969.
Mayo MW, Baldwin AS . The transcription factor NF-kappaB: control of oncogenesis and cancer therapy resistance. Biochim Biophys Acta 2000;1470:M55–M62.
Bottero V, Busuttil V, Loubat A, et al. Activation of nuclear factor kappaB through the IKK complex by the topoisomerase poisons SN38 and doxorubicin: a brake to apoptosis in HeLa human carcinoma cells. Cancer Res 2001;61:7785–7791.
Wang CY, Cusack Jr JC, Liu R, et al. Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-kappaB. Nat Med 1999;5:412–417.
Cusack Jr JC, Liu R, Houston M, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Res 2001;61:3535–3540.
Ariga A, Namekawa J, Matsumoto N, et al. Inhibition of tumor necrosis factor-alpha -induced nuclear translocation and activation of NF-kappa B by dehydroxymethylepoxyquinomicin. J Biol Chem 2002;277:24625–24630.
Matsumoto N, Ariga A, To-e S, et al. Synthesis of NF-kappaB activation inhibitors derived from epoxyquinomicin C. Bioorg Med Chem Lett 2000;10:865–869.
Horie R, Watanabe M, Ishida T, et al. The NPM-ALK oncoprotein abrogates CD30 signaling and constitutive NF-kappaB activation in anaplastic large cell lymphoma. Cancer Cell 2004;5:353–364.
Dewan MZ, Watanabe M, Terashima K, et al. Prompt tumor formation and maintenance of constitutive NF-kappaB activity of multiple myeloma cells in NOD/SCID/gammacnull mice. Cancer Sci 2004;95:564–568.
Cheng JD, Ryseck RP, Attar RM, et al. Functional redundancy of the nuclear factor kappa B inhibitors I kappa B alpha and I kappa B beta. J Exp Med 1998;188:1055–1062.
Huang TT, Miyamoto S . Postrepression activation of NF-kappaB requires the amino-terminal nuclear export signal specific to IkappaBalpha. Mol Cell Biol 2001;21:4737–4747.
Kim LH, Nadarajah VS, Peh SC, et al. Expression of Bcl-2 family members and presence of Epstein-Barr virus in the regulation of cell growth and death in classical Hodgkin's lymphoma. Histopathology 2004;44:257–267.
Dutton A, O'Neil JD, Milner AE, et al. Expression of the cellular FLICE-inhibitory protein (c-FLIP) protects Hodgkin's lymphoma cells from autonomous Fas-mediated death. Proc Natl Acad Sci USA 2004;101:6611–6616.
Thomas RK, Kallenborn A, Wickenhauser C, et al. Constitutive expression of c-FLIP in Hodgkin and Reed-Sternberg cells. Am J Pathol 2002;160:1521–1528.
Mathas S, Lietz A, Anagnostopoulos I, et al. c-FLIP mediates resistance of Hodgkin/Reed-Sternberg cells to death receptor-induced apoptosis. J Exp Med 2004;199:1041–1052.
Debatin KM . Apoptosis pathways in cancer and cancer therapy. Cancer Immunol Immunother 2004;53:153–159.
Piret B, Schoonbroodt S, Piette J . The ATM protein is required for sustained activation of NF-kappaB following DNA damage. Oncogene 1999;18:2261–2271.
Basu S, Rosenzweig KR, Youmell M, et al. The DNA-dependent protein kinase participates in the activation of NF kappa B following DNA damage. Biochem Biophys Res Commun 1998;247:79–83.
Zheng B, Georgakis GV, Li Y, et al. Induction of cell cycle arrest and apoptosis by the proteasome inhibitor PS-341 in Hodgkin disease cell lines is independent of inhibitor of nuclear factor-kappaB mutations or activation of the CD30, CD40, and RANK receptors. Clin Cancer Res 2004;10:3207–3215.
Izban KF, Ergin M, Huang Q, et al. Characterization of NF-kappaB expression in Hodgkin's disease: inhibition of constitutively expressed NF-kappaB results in spontaneous caspase-independent apoptosis in Hodgkin and Reed-Sternberg cells. Mod Pathol 2001;14:297–310.
Mathas S, Lietz A, Janz M, et al. Inhibition of NF-kappaB essentially contributes to arsenic-induced apoptosis. Blood 2003;102:1028–1034.
Acknowledgements
We thank Y Sato, National Institute of Infectious Diseases for technical assistance. Supported in part by Grants-in-Aid for Scientific Research from Japanese Society for Promotion of Science to R Horie and T Watanabe and Integrative Research Program of the Graduate School of Medical Sciences, Kitasato University to R Horie. Also supported in part by grants from the Ministry of Education, Science and Culture, Ministry of Health, Labor and Welfare and Human Health Science of Japan to N Yamamoto. Dr Kadin is supported by NIH Grant P50-CA-93683-05.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Watanabe, M., Dewan, M., Taira, M. et al. IκBα independent induction of NF-κB and its inhibition by DHMEQ in Hodgkin/Reed-Sternberg cells. Lab Invest 87, 372–382 (2007). https://doi.org/10.1038/labinvest.3700528
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/labinvest.3700528
Keywords
This article is cited by
-
A foretaste for pediatric glioblastoma therapy: targeting the NF-kB pathway with DHMEQ
Child's Nervous System (2023)
-
Combined effect of dehydroxymethylepoxyquinomicin and gemcitabine in a mouse model of liver metastasis of pancreatic cancer
Clinical & Experimental Metastasis (2013)