Abstract
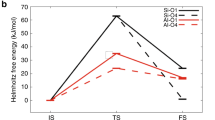
ZEOLITES are microporous aluminosilicates which, in their protonated form, act as solid catalysts1, and are widely used in the oil and petrochemical industries for processes such as cracking, isomerization and alkylation if hydrocarbons2. The proposed mechanisms3–5 of these processes mostly involve proton transfer and formation of carbenium or carbonium ions as reactive intermediates, but the detailed function of the zeolite and in particular the relation between acidity and catalytic activity is not well understood. Here we report experimental and theoretical studies of denterium–hydrogen exchange between deuterated methane and protonated zeolites — a prototypical hetero-geneous catalytic reaction between a hydrocarbon and an acid zeolite. We monitored this slow exchange reaction in two different zeolites using infrared spectroscopy, and used ab initio quantum chemistry calculations to determine both the reaction mechanism and the acidity–activity relationship. Combining our theoretical results with recent estimates8–11 of the acidity differences within zeolites enables us to reproduce the experimentally observed reaction rates and thus to obtain a detailed microscopic picture of this heterogeneous catalytic process.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Thomas, J. M. Scient. Am., 266 (IV), 82–88 (1992).
Maxwell, I. E. & Stork, W. H. J. in Introduction to Zeolite Science and Practice, (eds van Bekkum, H. et al.) 571–630 (Elsevier, Amsterdam, 1991).
Jacobs, P. A. & Martens, J. A. in Introduction to Zeolite Science and Practice. (eds van Bekkum, H. et al.) 445–496 (Elsevier, Amsterdam, 1991).
Abbot, J. & Wojciechowski, B. W. J. Catal. 115, 1–15 (1989).
Stefanidis, C., Gates, B. C. & Haag, W. O. J. Molec. Catal. 67, 363–367 (1991).
Sauer, J., Kölmel, C. M., Hill, J. R. & Ahlrichs, R. Chem. Phys. Lett. 164, 193–198 (1989).
Sauer, J., Horn, H., Häser, M. & Ahlrichs, R. Chem. Phys. Lett. 173, 26–32 (1990).
Dubsky, J. Beran, S. & Bosác̆ek, V. J. Molec. Catal. 6, 321–326 (1979).
Schröder, K.-P., Sauer, J., Leslie, M., Catlow, R. C. A. & Thomas, J. M. Chem. Phys. Lett. 188, 320–325 (1992).
Schröder, K.-P., Sauer, J., Leslie, M. & Catlow, R. C. A. Zeolites 12, 20–23 (1992).
Kramer, G. J. & van Santen, R. A. J. Am. chem. Soc. 115, 2887–2897 (1993).
Kramer, G. J., de Man, A. J. M. & van Santen, R. A. J. Am. chem. Soc. 113, 6435–6441 (1991).
Sauer, J. chem. Rev. 89, 199–255 (1989).
Dupuis, M., Sprangler, D. & Wendolowski, D. NRCC Software Catalog 1, Program No. QG01, GAMESS (1980).
Szabo, A. & Ostlund, N. S. Modern Quantum Chemistry: Introduction to Advanced Electronic Structure Theory (Macmillan, New York, 1982).
Rice, O. K. Statistical Mechanics, Thermodynamics and Kinetics (Freeman, San Francisco, 1966).
Thruhlar, D. G. & Gordon, M. S. Science 249, 491–497 (1990).
Gilbert, R. G. & Smith, S. C. Theory of Unimolecular and Recombination Reactions (Blackwell, Oxford, 1990).
Barrer, R. M., & Sutherland, J. W. Proc. R. Soc. A237, 439–450 (1956).
Papp, H., Hinsen, W., Do, N. T. & Baerns, M., Thermochim. Acta 82, 137–148 (1984).
Yashonath, S., Thomas, J. M., Nowak, A. K. & Cheetham, A. K. Nature 331, 601–604 (1988).
Johnston, H. S. Gas Phase Reaction Rate Theory (Ronald, New York, 1966).
Meier, W. M. & Olson, D. H. Atlas of Zeolite Structure Types, 2nd edn (Butterworth, Cambridge, 1987).
Lombardo, E. A., Sill, G. A. & Hall, W. K. J. Catal. 119, 426–440 (1989).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Kramer, G., van Santen, R., Emeis, C. et al. Understanding the acid behaviour of zeolites from theory and experiment. Nature 363, 529–531 (1993). https://doi.org/10.1038/363529a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/363529a0
This article is cited by
-
Direct assessment of the acidity of individual surface hydroxyls
Nature (2021)
-
A Comparative Study of n-Butane Isomerization over H-Beta and H-ZSM-5 Zeolites at Low Temperatures: Effects of Acid Properties and Pore Structures
Catalysis Letters (2019)
-
Rearrangement of Cyclopropylcarbinyl Chloride Over Protonic Zeolites: Formation of Carbocations and Behavior as Solid Solvents
Topics in Catalysis (2018)
-
Mechanisms of alkylation of isobutane by butenes and H/D exchange in isobutane molecules on acid zeolites
Theoretical and Experimental Chemistry (2013)
-
Proton exchange reactions of C2–C4 alkanes sorbed in ZSM-5 zeolite
Theoretical Chemistry Accounts (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.