Abstract
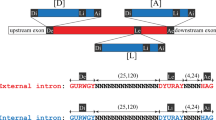
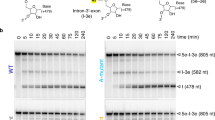
Group I, group II and spliceosomal introns splice by two sequential transesterification reactions1. For both spliceosomal and group II introns, the first-step reaction occurs by nucleophilic attack on the 5′ splice junction by the 2′ hydroxyl of an internal adenosine, forming a 2′–5′ phosphodiester branch in the intron. The second reaction joins the two exons with a 3′–5′ phosphodiester bond and releases intron lariat. In vitro, group II introns can self-splice by an efficient alternative pathway in which the first-step reaction occurs by hydrolysis. The resulting linear splicing intermediate participates in normal second-step reactions, forming spliced exon and linear intron RNAs2,3. Here we show that the group II intron first-step hydrolysis reaction occurs in vivo in place of transesterification in the mitochondria of yeast strains containing branch-site mutations. As expected, the mutations block branching, but surprisingly still allow accurate splicing. This hydrolysis pathway may have been a step in the evolution of splicing mechanisms.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Cech, T. R. in The RNA World (eds Gesteland, R. F. & Atkins, J. F.) 239–270 (Cold Spring Harbor Laboratory Press, NY, 1993).
Jarrell, K. A., Peebles, C. L., Dietrich, R. C., Romiti, S. L. & Perlman, P. S. Group II intron self-splicing: alternative reaction conditions yield novel products. J. Biol. Chem. 263, 3432–3439 (1988).
Daniels, D., Michels, W. J. & Pyle, A. M. Two competing pathways for self-splicing by group II introns: a quantitative analysis of in-vitro reaction rates and products. J. Mol. Biol. 256, 31–49 (1996).
Michel, F. & Ferat, J. L. Structure and activities of group II introns. Annu. Rev. Biochem. 64, 435–461 (1995).
Liu, Q.et al. Branch-site selection in a group II intron mediated by active recognition of the adenine amino group and steric exclusion of non-adenine functionalities. J. Mol. Biol. 267, 163–171 (1997).
Arnberg, A., Ommen, G. V., Grivell, L., Bruggen, E. V. & Borst, P. Some yeast mitochrondrial RNAs are circular. Cell 19, 313–319 (1980).
Hensgens, L. A. M.et al. Variation, transcription, and circular RNAs of the mitochondrial gene for subunit 1 of cytochrome c oxidase. J. Mol. Biol. 164, 35–58 (1983).
Contrad-Webb, H., Perlman, P. S., Zhu, H. & Butow, R. A. The nuclear SUV3-1 mutation affects a variety of post-transcriptional processes in yeast mitochondria. Nucleic Acids Res. 18, 1369–1376 (1990).
Margossian, S., Li, H., Zassenhaus, H. & Butow, R. The DExH box protein Suv3p is a component of a yeast mitochondrial 3′-to-5′ exoribonuclease that suppresses group I intron toxicity. Cell 84, 199–209 (1996).
Margossian, S. & Butow, R. RNA turnover and the control of mitochondrial gene expression. Trends Biochem. Sci. 21, 392–396 (1996).
Peebles, C. L., Belcher, S. M., Zhang, M., Dietrich, R. C. & Perlman, P. S. Mutation of the conserved first nucleotide of a group II intron from yeast mitochondrial DNA reduces the rate but allows accurate splicing. J. Biol. Chem. 268, 11929–11938 (1993).
Boulanger, S. C.et al. Length changes in the joining segment between domains 5 and 6 of a group II intron inhibit self-splicing and alter 3′ splice site selection. Mol. Cell. Biol. 16, 5896–5904 (1996).
Costanzo, M. C. & Fox, T. D. Control of mitochondrial gene expression in Saccharomyces cerevisiae. Annu. Rev. Genet. 24, 91–113 (1990).
Jacquier, A. & Jacquesson-Breuleux, N. Splice site selection and role of the lariat in a group II intron. J. Mol. Biol. 219, 415–428 (1991).
Chanfreau, G. & Jacquier, A. Interaction of intronic boundaries is required for the second splicing step efficiency of a group II introns. EMBO J. 12, 5173–5180 (1993).
Chapman, K. B. & Boeke, J. D. Isolation and characterization of the gene encoding yeast debranching enzyme. Cell 65, 483–492 (1991).
Michel, F., Umesono, K. & Ozeki, H. Comparative and functional anatomy of group II catalytic introns—a review. Gene 82, 5–30 (1989).
Eskes, R., Yang, J., Lambowitz, A. & Perlman, P. Mobility of yeast mitochondrial group II introns: engineering a new site specificity and retrohoming via full reverse splicing. Cell 88, 865–874 (1997).
Zimmerly, S.et al. Agroup II intron RNA is a catalytic component of a DNA endonuclease involved in intron mobility. Cell 83, 529–538 (1995).
Butow, R. A., Belcher, S. M., Moran, J. V., Henke, R. M. & Perlman, P. S. Transformation of S. cerevisiae mitochondria using the biolistic gun. Methods Enzymol. 264, 265–278 (1995).
Boulanger, S. C.et al. Studies of point mutants define essential nucleotides in the domain 5 substructure of a group II intron. Mol. Cell Biol. 15, 4479–4488 (1995).
Moran, J. V.et al. Splicing defective mutants of the COXI gene of yeast mitochondrial DNA: Initial definition of the maturase domain of the group II intron AI2. Nucleic Acids Res. 22, 2057–2064 (1994).
Goda, S. K. & Minton, N. P. Asimple procedure for gel electrophoresis and Northern blotting of RNA. Nucleic Acids Res. 23, 3357–3358 (1995).
Peebles, C. L.et al. Aself-splicing RNA excises an intron lariat. Cell 44, 213–223 (1986).
Podar, M., Dib-Hajj, S. & Perlman, P. S. AUV-induced, Mg2+-dependent crosslink traps an active form of domain 3 of a self-splicing group II intron. RNA 1, 828–840 (1995).
Perlman, P. & Podar, M. Reactions catalyzed by group II introns in vitro. Methods Enzymol. 264, 66–86 (1995).
Bonitz, S. G., Coruzzi, G., Thalenfield, B. E., Tzagoloff, A. & Macino, G. Assembly of the mitochondrial membrane system. Structure and nucleotide sequence of the gene coding for subunit I of cytochrome oxidase. J. Biol. Chem. 255, 11927–11941 (1980).
Acknowledgements
This work was supported by NIH grants to P.S.P. and A.M.P. M.P. was a fellow of the Robert A. Welch Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Podar, M., Chu, V., Pyle, A. et al. Group II intron splicing in vivo by first-step hydrolysis. Nature 391, 915–918 (1998). https://doi.org/10.1038/36142
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/36142
This article is cited by
-
Sequential splicing of a group II twintron in the marine cyanobacterium Trichodesmium
Scientific Reports (2015)
-
Biotechnological applications of mobile group II introns and their reverse transcriptases: gene targeting, RNA-seq, and non-coding RNA analysis
Mobile DNA (2014)
-
Lariat lessons
Nature (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.