Abstract
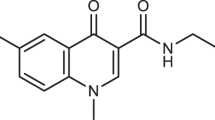
HUMAN cytomegalovirus (HCMV, a betaherpes virus) is the cause of serious disease in immunologically compromised individuals, including those with acquired immunodeficiency syndonie1. One of the compounds used in the chemotherapy of HCMV infections is the nucleoside analogue 9-(l,3-dihydroxy-2-propoxymethyl)-guanine (ganciclovir). The mechanism of action of this drug is dependent on the formation of the nucleoside triphosphate, which is a strong inhibitor of the viral DNA polymerase2–4. Thymidine kinase, which is encoded by many of the herpesviruses, catalyses the initial phosphorylation of ganciclovir. But there is no evidence for the coding of this enzyme by HCMV2,5,6, and DNA sequence analysis of the HCMV genome has shown that there is no open reading frame characteristic of a herpesvirus thymidine kinase7. Here we present biochemical and immunological evidence that the HCMV UL97 open reading frame codes for a protein capable of phosphorylating ganciclovir. This protein seems to be responsible for the selectivity of ganciclovir and will be useful tool in the understanding and refinement of the antiviral activity of new selective anti-HCMV compounds.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Macher, A. M. et al. New Eng. J. Med. 309, 1454 (1983).
Biron, K. K. et al. Proc. natn. Acad. Sci. U.S.A. 82, 2473–2477 (1985).
Biron, K. K. et al. Proc. natn. Acad. Sci. U.S.A. 83, 7769–8773 (1986).
Freitas, V. R., Smee, D. F., Chernow, M., Boehme, R. & Matthews, T. R. Antimicrob. Ag. Chemother. 28, 240–245 (1985).
Estes, J. E. & Huang, E. S. J. Virol. 24, 13–21 (1976).
Fyfe, J. A., Keller, P. M., Furman, P. A., Miller, R. L. & Elion, G. B. L. J. biol. Chem. 253, 8721–8727 (1978).
Chee, M. S. et al. Curr. Top. Microbiol. Immun. 154, 125–169 (1990).
Chee, M. S., Lawrence, G. L. & Barrell, B. G. J. gen. Virol. 70, 1151–1160 (1989).
Edelman, A. M., Blumenthal, D. K. & Krebs, E. G. A. Rev. Biochem. 56, 567–613 (1987).
Pearson, R. B., Wettenhall, R. E. H., Means, A. R., Hartshorne, D. J. & Kemp, B. E. Science 241, 970–973 (1988).
Rosenberg, A. H. et al. Gene 56, 31–38 (1987).
Ertl, P. F., Thomas, M. S. & Powell, K. L. J. gen. Virol. 72, 1729–1734 (1991).
Sullivan, V. et al. Nature 358, 162–164 (1992).
Stanat, S. C. et al. Antimicrob. Ag. Chemother. 35, 2191–2197 (1991).
Smith, R. F. & Smith, T. F. J. J. Virol. 63, 450–455 (1989).
Knighton, D. R. et al. Science 253, 414–420 (1991).
Littler, E., Lawrence, G., Liu, M.-Y., Barrell, B. G. & Arrand, J. R. J. Virol. 64, 714–722 (1990).
Littler, E. & Arrand, J. R. J. Virol. 62, 3892–3895 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Littler, E., Stuart, A. & Chee, M. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature 358, 160–162 (1992). https://doi.org/10.1038/358160a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/358160a0
This article is cited by
-
Nucleoside analogs as potential antiviral agents for dengue virus infections
Medicinal Chemistry Research (2017)
-
Cytomegalovirus infection in immunocompetent critically ill adults: literature review
Annals of Intensive Care (2016)
-
Liquid chromatography tandem mass spectrometry quantitation of intracellular concentrations of ganciclovir and its phosphorylated forms
Analytical and Bioanalytical Chemistry (2015)
-
Pharmacokinetics and Pharmacodynamics of Antibacterials, Antifungals, and Antivirals Used Most Frequently in Neonates and Infants
Clinical Pharmacokinetics (2014)
-
Successful ganciclovir treatment of primary cytomegalovirus infection containing the UL97 mutation N510S in an intestinal graft recipient
Infection (2013)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.