Key Points
-
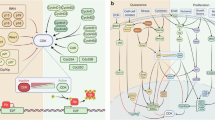
The cell cycle is regulated by cyclin-dependent kinases (CDKs), which form active heterodimeric kinases when bound to the cyclins. Active CDK–cyclin complexes can be negatively regulated by two families of inhibitors, the INK4 and the WAF1/KIP proteins.
-
The CDK4/6–cyclin-D and CDK2–cyclin-E complexes are active in G1, and CDK2–cyclin-A and CDK1–cyclin-A are active in S phase. The CDK complex responsible for driving cells through mitosis is CDK1–cyclin B.
-
The main G1 substrates of CDKs are the retinoblastoma family (RB, p107 and p130). RB is sequentially phosphorylated by CDK4/6–cyclin-D and CDK2–cyclin-E complexes. This phosphorylation inactivates the growth-suppression properties of RB and stimulates progression through G1 and into S phase.
-
The basic regulators of G1 progression are altered in most human cancers. Genetic alterations usually affect CDK4 and CDK6, their positive (mainly cyclin D1) and negative (INK4A and INK4B) regulators and their substrates (mainly RB).
-
Deregulation of CDK2 activity frequently results from the alteration in the expression levels of its regulators cyclin E and KIP1. The cause of these alterations is not clear. Recently, some members of the proteolysis pathways involved in the control of cyclin E and KIP1 protein levels have been found to be altered in certain types of cancer.
-
A few other cell-cycle regulators, mostly those involved in the mitotic spindle checkpoint, are also deregulated in human cancer. They include BUB proteins, MAD2, the Aurora kinases, PLK1 and securin. The evidence for their involvement in human cancer is, so far, scarce.
-
The generation of gene-targeted mice has illustrated the importance of G1 regulators in tumour development. Although most of these are not essential for cell-cycle progression or mouse development, their deregulation often leads to tumour development.
-
The understanding of G1 regulation has opened new avenues to search for antitumor drugs. Although there are several ways to control CDK activity, small-molecule CDK inhibitors are the preferred tools, at this time. So far, several CDK inhibitors (mainly CDK2 and pan-CDK inhibitors) are in advanced preclinical studies and at least two of them — flavopiridol and UCN-01 — have reached clinical trials.
Abstract
Tumour cells undergo uncontrolled proliferation, yet tumours most often originate from adult tissues, in which most cells are quiescent. So, the proliferative advantage of tumour cells arises from their ability to bypass quiescence. This can be due to increased mitogenic signalling and/or alterations that lower the threshold required for cell-cycle commitment. Understanding the molecular mechanisms that underlie this commitment should provide important insights into how normal cells become tumorigenic and how new anticancer strategies can be devised.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Norbury, C. & Nurse, P. Animal cell cycles and their control. Annu. Rev. Biochem. 61, 441–470 (1992).
Hartwell, L. & Weinert, T. Checkpoints: controls that ensure the order of cell cycle events. Science 246, 629–634 (1989).
Morgan, D. O. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu. Rev. Cell Dev. Biol. 13, 261–291 (1997).
Sherr, C. J. Cancer cell cycles revisited. Cancer Res. 60, 3689–3695 (2000).
Meyerson, M. & Harlow, E. Identification of G1 kinase activity for cdk6, a novel cyclin D partner. Mol. Cell. Biol. 14, 2077–2086 (1994).
Jinno, S. et al. Oncogenic stimulation recruits cyclin-dependent kinase in the cell cycle start in rat fibroblast. Proc. Natl Acad. Sci. USA 96, 13197–13202 (1999).
Rane, S. G. et al. Loss of Cdk4 expression causes insulin-deficient diabetes and Cdk4 activation results in B-cell hyperplasia. Nature Genet. 22, 44–52 (1999).
Tsutsui, T. et al. Targeted disruption of Cdk4 delays cell cycle entry with enhanced p27Kip1 activity. Mol. Cell. Biol. 19, 7011–7019 (1999).References 7 and 8 describe the generation of mice that are deficient for Cdk4. Cdk4 seems to be dispensable for the cell cycle in most cell types. Ablation of Cdk4, however, severely affects proliferation of specific cell types, such as pancreatic β-cells, which leads to diabetes.
Ye, X., Zhu, C. & Harper, W. A premature-termination mutation in the Mus musculus cyclin-dependent kinase 3 gene. Proc. Natl Acad. Sci. USA 13, 1682–1686 (2001).
Nigg, E. A. Cyclin-dependent kinase 7: at the cross-roads of transcription, DNA repair and cell cycle control? Curr. Opin. Cell Biol. 8, 312–317 (1996).
Ekholm, S. V. & Reed, S. I. Regulation of G1 cyclin-dependent kinases in the mammalian cell cycle. Curr. Opin. Cell Biol. 12, 676–684 (2000).
Malumbres, M. & Pellicer, A. Ras pathways to cell cycle control and cell transformation. Front. Biosci. 3, D887–D912 (1998).
Diehl, J. A., Cheng, M., Roussel, M. F. & Sherr, C. J. Glycogen synthase kinase-3β regulates cyclin D1 proteolysis and subcellular localization. Genes Dev. 12, 3499–3511 (1998).
Alt, J. R., Cleveland, J. L., Hannink, M. & Diehl, J. A. Phosphorylation-dependent regulation of cyclin D1 nuclear export and cyclin D1-dependent cellular transformation. Genes Dev. 14, 3102–3114 (2000).
Fantl, V., Stamp, G., Andrews, A., Rosewell, I. & Dickson, C. Mice lacking cyclin D1 are small and show defects in eye and mammary gland development. Genes Dev. 9, 2364–2372 (1995).
Sicinski, P. et al. Cyclin D1 provides a link between development and oncogenesis in the retina and breast. Cell 82, 621–630 (1995).
Sherr, C. J. & Roberts, J. M. Cdk inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13, 1501–1512 (1999).
Adams, P. D. Regulation of the retinoblastoma tumor suppressor protein by cyclin/CDKs. Biochim. Biophys. Acta 1471, 123–133 (2001).
Harbour, J. W. & Dean, D. C. The pRb/E2F pathway: expanding roles and emerging paradigms. Genes Dev. 14, 2393–2409 (2000).
Morris, E. J. & Dyson, N. J. Retinoblastoma protein partners. Adv. Cancer Res. 82, 1–54 (2001).
Lundberg, A. S. & Weinberg, R. A. Functional inactivation of the retinoblastoma protein requires sequential modification by at least two distinct cyclin–CDK complexes. Mol. Cell. Biol. 18, 753–761 (1998).
Harbour, J. W., Luo, R. X., Dei Santi, A., Postigo, A. A. & Dean, D. C. Cdk phosphorylation triggers sequential intramolecular interactions that progressively block Rb functions as cells move through G1. Cell 98, 859–869 (1999).
Ezhevsky, S. A., Ho, A., Becker-Hapak, M., Davis, P. K. & Dowdy, S. F. Differential regulation of retinoblastoma tumor suppressor protein by G1 cyclin-dependent kinase complexes in vivo. Mol. Cell. Biol. 21, 4773–4784 (2001).References 21–23 culminate a series of reports that describe the distinct effects of cyclin- d -dependent and cyclin-E-dependent CDKs on the RB protein. The different role of these kinases in RB regulation provides us with interesting insights regarding the regulation of the early G1 and late G1 phase.
Chan, H. M., Krstic-Demonacos, M., Smith, L., Demonacos, C. & La Thangue, N. B. Acetylation control of the retinoblastoma tumour-suppressor protein. Nature Cell Biol. 3, 667–674 (2001).
Tamrakar, S., Rubin, E. & Ludlow, J. W. Role of pRb dephosphorylation in cell cycle regulation. Front. Biosci. 5, D121–D137 (2000).
Van den Heuvel, M. & Harlow, E. Distinct roles for cyclin-dependent kinases in cell cycle control. Science 262, 2050–2054 (1993).This study provided evidence for a distinct role of the different CDKs in G1/S progression. Whereas dominant-negative forms of CDK2 or CDK3 can arrest cultured cells in G1, similar mutant forms of CDK4 or CDK6 cannot.
Zhang, H. S., Postigo, A. A. & Dean, D. C. Active transcriptional repression by the Rb–E2F complex mediates G1 arrest triggered by p16INK4a, TGFβ, and contact inhibition. Cell 97, 53–61 (1999).
Blain, S. W., Montalvo, E. & Massagué, J. Differential interaction of the cyclin-dependent kinase (Cdk) inhibitor p27Kip1 with cyclin-A–Cdk2 and cyclin-D2–Cdk4. J. Biol. Chem. 272, 25863–25872 (1997).
LaBaer, J. et al. New functional activities for the p21 family of CDK inhibitors. Genes Dev. 11, 847–862 (1997).
Cheng, M. et al. The p21Cip1 and p27Kip1 Cdk inhibitors are essential activators of cyclin D-dependent kinases in murine fibroblasts. EMBO J. 18, 1571–1583 (1999).References 28–30 illustrate the differential consequences of the binding of WAF1/KIP inhibitors to CDK4/6–cyclin-D and CDK2–cyclin-E/A complexes.
Geng, Y. et al. Rescue of cyclin D1 deficiency by knockin cyclin E. Cell 97, 767–777 (1999).Replacement of cyclin D1 by a human cyclin E1 cDNA restores most of the deficiencies of cyclin-D1 knockout mice. As cyclin E cannot activate CDK4 or CDK6, these observations indicate that cyclin E1 is a main downstream target of cyclin D1.
Geng, Y. et al. Deletion of the p27Kip1 gene restores normal development in cyclin D1-deficient mice. Proc. Natl Acad. Sci. USA, 98, 194–199 (2001).
Tong, W. & Pollard, J. W. Genetic evidence for the interactions of cyclin D1 and p27Kip1 in mice. Mol. Cell. Biol. 21, 1319–1328 (2001).
Sheaff, R. J., Groudine, M., Gordon, M., Roberts, J. M. & Clurman, B. E. Cyclin E-CDK2 is a regulator of p27Kip1. Genes Dev. 11, 1464–1478 (1997).
Malek, N. P. et al. A mouse knock-in model exposes sequential proteolytic pathways that regulate p27Kip1 in G1 and S phase. Nature 413, 323–327 (2001).Cells that express a knock-in Kip1 T187A mutant are unable to downregulate Kip1 during S and G2. However, this mutation does not affect Kip1 downregulation in G1, indicating the existence of an alternative degradation process.
Moberg, K. H., Bell, D. W., Wahrer, D. C. R., Haber, D. A. & Hariharan, I. K. Archipielago regulates cyclin E levels in Drosophila and is mutated in human cancer cell lines. Nature 413, 311–316 (2001).
Strohmaier, H. et al. Human F-box protein hCdc4 targets cyclin E for proteolysis and is mutated in a breast cancer cell line. Nature 413, 316–322 (2001).
Koepp, D. M. et al. Phosphorylation-dependent ubiquitination of cyclin E by the SCFFbw7 ubiquitin ligase. Science 294, 173–177 (2001).
Wolfel, T. et al. A p16Ink4a-insensitive Cdk4 mutant targeted by cytolytic T lymphocytes in a human melanoma. Science 269, 1281–1284 (1995).First description of a tumour-associated point mutation in a CDK molecule. Interestingly, this CDK4 mutation (R24C) disrupts the binding with the INK4 family of inhibitors, highlighting the importance of this interaction in tumour development.
Easton, J., Wei, T., Lahti, J. M. & Kidd, V. J. Disruption of the cyclin D/cyclin-dependent kinase/INK4/retinoblastoma protein regulatory pathway in human neuroblastoma. Cancer Res. 58, 2624–2632 (1998).
Latres, E. et al. Role of the F-box protein Skp2 in lymphomagenesis. Proc. Natl Acad. Sci. USA 98, 2515–2520 (2001).
Gstaiger, M. et al. Skp2 is oncogenic and overexpressed in human cancers. Proc. Natl Acad. Sci. USA 98, 5043–5048 (2001).
Wu, C.-L. et al. Cables enhances Cdk2 tyrosine 15 phosphorylation by Wee1, inhibits cell growth, and is lost in many human colon and squamous cancers. Cancer Res. 61, 7325–7332 (2001).
Paggi, M. G. & Giordano, A. Who is the boss in the retinoblastoma family? The point of view of Rb2/p130, the little brother. Cancer Res. 61, 4651–4654 (2001).
Lee, E. Y. H. P. et al. Mice deficient for Rb are nonviable and show defects in neurogenesis and hematopoiesis. Nature 359, 288–294 (1992).
Jacks, T. et al. Effects of an Rb mutation in the mouse. Nature 359, 295–300 (1992).
Mulligan, G. & Jacks, T. The retinoblastoma gene family: cousins with overlapping interests. Trends Genet. 14, 223–229 (1998).
Robanus-Maandag, E. et al. p107 is a suppressor of retinoblastoma development in pRb-deficient mice. Genes Dev. 12, 1599–1609 (1998).
Fero, M. L. et al. A syndrome of multiorgan hyperplasia with features of gigantism, tumorigenesis, and female sterility in p27Kip1-deficient mice. Cell 85, 733–744 (1996).
Kiyokawa, H. et al. Enhanced growth of mice lacking the cyclin-dependent kinase inhibitor function of p27Kip1. Cell 85, 721–732 (1996).
Nakayama, K. et al. Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85, 707–720 (1996).
Fero, M. L., Randel, E., Gurley, K. E., Roberts, J. M. & Kemp, C. J. The murine gene p27Kip1 is haplo-insufficient for tumour suppression. Nature 396, 177–180 (1998).
Martin-Caballero, J., Flores, J. M., Garcia-Palencia, P. & Serrano, M. Tumor susceptibility of p21(Waf1/Cip1)-deficient mice. Cancer Res. 61, 6234–6238 (2001).
Krimpenfort, P., Quon, K. C., Mooi, W. J., Loonstra, A. & Berns, A. Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature 413, 83–86 (2001).
Sharpless, N. E. et al. Loss of p16INK4a with retention of p19ARF predisposes to tumorigenesis. Nature 413, 86–91 (2001).References 54 and 55 define the specific role of INK4A as a tumour suppressor. They also highlight that loss of INK4A increases predisposition for melanoma, a neoplasia in which the CDKN2A (which encodes INK4A and ARF) region was first described as a susceptibility locus (see also reference 59).
Latres, E. et al. Limited overlapping roles of p15INK4b and p18INK4c cell cycle inhibitors in proliferation and tumorigenesis. EMBO J. 19, 3496–3506 (2000).
Franklin, D. S. et al. Cdk inhibitors p18Ink4c and p27Kip1 mediate two separate pathways to collaborative suppress pituitary tumorigenesis. Genes Dev. 12, 2899–2911 (1998).
Sotillo, R. et al. Wide spectrum of tumors in knock-in mice carrying a Cdk4 protein insensitive to INK4 inhibitors. EMBO J. (in the press).
Sotillo, R. et al. Invasive melanoma in Cdk4 targeted mice. Proc. Natl Acad. Sci. USA 98, 13312–13317 (2001).
Wang, T. C. et al. Mammary hyperplasia and carcinoma in MMTV-cyclin D1 transgenic mice. Nature 369, 669–671 (1994).These studies (references 58–60 ) illustrate the role of CDK4–cyclin-D misregulation in tumour development and tumour susceptibility.
Bortner, D. M. & Rosenberg, M. P. Induction of mammary gland hyperplasia and carcinomas in transgenic mice expressing human cyclin E. Mol. Cell. Biol. 17, 453–459 (1997).
Brugarolas, J., Bronson, R. T. & Jacks, T. p21 is a critical regulator essential for proliferation control in Rb-deficient cells. J. Cell Biol. 141, 503–514 (1998).
Park, M. S. et al. p27 and Rb are on overlapping pathways suppressing tumorigenesis in mice. Proc. Natl Acad. Sci. USA 96, 6382–6387 (1999).
Franklin, D. S., Godfrey, V. L., O'Brien, D. A., Deng, C. & Xiong, Y. Functional collaboration between different cyclin-dependent kinase inhibitors suppresses tumor growth with distinct tissue specificity. Mol. Cell. Biol. 20, 6147–6158 (2000).
Chen, Y.-N. P. et al. Selective killing of transformed cells by cyclin/cyclin-dependent kinase 2 antagonists. Proc. Natl Acad. Sci. USA 96, 4325–4329 (1999).
Blagosklonny, M. V. & Pardee, A. B. Exploiting cancer cell cycling for selective protection of normal cells. Cancer Res. 61, 4301–4305 (2001).
Noble, M. E. M. & Endicott, J. A. Chemical inhibitors of cyclin-dependent kinases: insights into design from X-ray crystallographic studies. Pharmacol. Ther. 82, 269–278 (1999).
Sandig, V. et al. Adenovirally transferred p16INK4a/CDKN2 and p53 genes cooperate to induce apoptotic tumor cell death. Nature Med. 3, 313–319 (1997).
Fahraeus, R., Lain, S., Ball, K. L. & Lane, D. P. Characterization of the cyclin-dependent kinase inhibitory domain of the INK4 family as a model for a synthetic tumour suppressor molecule. Oncogene 16, 587–596 (1998).
Lubbert, M. DNA methylation inhibitors in the treatment of leukemias, myelodysplastic syndromes and hemoglobinopathies: clinical results and possible mechanisms of action. Curr. Top. Microbiol. Immunol. 249, 135–164 (2000).
Adams, J. et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Cancer Res. 59, 2615–2622 (1999).
Arber, N. et al. Antisense to cyclin D1 inhibits the growth and tumorigenicity of human colon cancer cells. Cancer Res. 57, 1569–1574 (1997).
Pestell, K. E., Ducruet, A. P., Wipf, P. & Lazo, J. S. Small molecule inhibitors of dual specificity protein phosphatases. Oncogene 19, 6607–6612 (2000).
Pavletich, N. P. Mechanisms of cyclin-dependent kinase regulation: structures of Cdks, their cyclin activators and Cip and Ink inhibitors. J. Mol. Biol. 287, 821–828 (1999).
Gray, N. S. et al. Exploiting chemical libraries, structure, and genomics in the search for kinase inhibitors. Science 281, 533–538 (1998).An example of the synergistic combination of in vitro and in silico approaches in the search for CDK inhibitors of therapeutic value.
Lander et al. Initial sequencing and analysis of the human genome. Nature 409, 860–921 (2001).
Garrett, M. D. & Fattaey, A. CDK inhibition and cancer therapy. Curr. Opin. Genet. Dev. 9, 104–111 (1999).
Crews, C. M. & Mohan, R. Small-molecule inhibitors of the cell cycle. Curr. Opin. Chem. Biol. 4, 47–53 (2000).
Hoessel, R. et al. Indirubin, the active constituent of a Chinese antileukaemia medicine, inhibits cyclin-dependent kinases. Nature Cell Biol. 1, 60–67 (1999).Indirubin can specifically inhibit CDKs and arrest cell-cycle progression. An example of how classical Chinese medicine might help to identify inhibitors of molecular targets.
Senderowicz, A. M. & Sausville, E. A. Preclinical and clinical development of cyclin-dependent kinase modulators. J. Natl Cancer Inst. 92, 376–387 (2000).
Brooks, G. & La Thangue, N. B. The cell cycle and drug discovery: the promise and the hope. Drug Discovery Today 4, 455–464 (1999).
Meijer, L., Leclerc, S. & Leost, M. Properties and potential applications of chemical inhibitors of cyclin-dependent kinases. Pharmacol. Ther. 82, 279–284 (1999).
Wood, K. W., Cornwell, W. D. & Jackson, J. R. Past and future of the mitotic spindle as an oncology target. Curr. Opin. Pharmacol. 1, 370–377 (2001).
Altmann, K.-H. Microtubule-stabilizing agents: a growing class of important anticancer drugs. Curr. Opin. Chem. Biol. 5, 424–431 (2001).
Thomas, G. An encore for ribosome biogenesis in the control of cell proliferation. Nature Cell. Biol. 2, E71–E72 (2000).
Schmelzle, T. & Hall, M. N. TOR, a central controller of cell growth. Cell 103, 253–262 (2000).
Rohde, J., Heitman, J. & Cardenas, M. E. The TOR kinases link nutrient sensing to cell growth. J. Biol. Chem. 276, 9583–9586 (2001).
Fukunaga, R. & Hunter, T. MNK1, a new MAP kinase-activated protein kinase, isolated by a novel expression screening method for identifying protein kinase substrates. EMBO J. 16, 1921–1933 (1997).
Waskiewicz, A. J., Flynn, A., Proud, C. G. & Cooper, J. A. Mitogen-activated protein kinases activate the serine/threonine kinases Mnk1 and Mnk2. EMBO J. 16, 1909–1920 (1997).
Pardee, A. B. A restriction point for control of normal animal cell proliferation. Proc. Natl Acad. Sci. USA 71, 1286–1290 (1974).
Planas-Silva, M. D. & Weinberg, R. A. The restriction point and control of cell proliferation. Curr. Opin. Cell Biol. 9, 768–772 (1997).
Dannenberg, J.-H., van Rossum, A., Schuijff, L. & te Riele, H. Ablation of the retinoblastoma gene family deregulates G1 control causing immortalization and increased cell turnover under growth-restricting conditions. Genes Dev. 14, 3051–3064 (2000).
Sage, J. et al. Targeted disruption of the three Rb-related genes leads to loss of G1 control and immortalization. Genes Dev. 14, 3037–3050 (2000).References 92 and 93 illustrate the specific and redundant roles of the RB protein family members in regulating the cell cycle.
Park, M. & Krause, M. W. Regulation of postembryonic G1 cell cycle progression in Caenorhabditis elegans by a cyclin D/CDK-like complex. Development 126, 4849–4860 (1999).
Meyer, C. A. et al. Drosophila Cdk4 is required for normal growth and is dispensable for cell cycle progression. EMBO J. 19, 4533–4542 (2000).Shows that in Drosophila , the single cyclin- d -dependent Cdk (equivalent to mammalian CDK4 and CDK6) is not required for cell proliferation but affects homeotic cell number.
Datar, S. A., Jacobs, H. W., de la Cruz, A. F., Lehner, C. F. & Edgar, B. A. The Drosophila cyclin-D–Cdk4 complex promotes cellular growth. EMBO J. 19, 4543–4554 (2000).
Yu, Q., Geng, Y. & Sicinski, P. Specific protection against breast cancers by cyclin D1 ablation. Nature 411, 1017–1021 (2001).Genetic analysis of the relationship between the tumorigenic properties of specific oncogenes and cyclin D1 in breast cancer. This report exemplifies the use of genetic models to shape specific pathways for therapeutic intervention in human cancer.
Shah, J. V. & Cleveland, D. W. Waiting for anaphase: Mad2 and the spindle assembly checkpoint. Cell 103, 997–1000 (2000).
Nigg, E. A. Cell division mitotic kinases as regulators of cell division and its checkpoints. Nature Rev. Mol. Cell. Biol. 2, 21–32 (2001).
Cahill, D. P. et al. Mutations of mitotic checkpoint genes in human cancers. Nature 392, 300–303 (1998).
Michel, L. S. et al. MAD2 haplo-insufficiency causes premature anaphase and chromosome instability in mammalian cells. Nature 409, 355–359 (2001).First genetic demonstration that defects in the mitotic checkpoint can lead to tumour development.
Hernando, E. et al. Molecular analyses of the mitotic checkpoint components hsMAD2, hBUB1 and hBUB3 in human cancer. Int. J. Cancer 95, 223–227 (2001).
Smith, M. R. et al. Malignant transformation of mammalian cells initiated by constitutive expression of the polo-like kinase. Biochem. Biophys. Res. Commun. 234, 397–405 (1997).
Simizu, S. & Osada, H. Mutations in the Plk gene lead to instability of Plk protein in human tumour cell lines. Nature Cell. Biol. 2, 852–854 (2000).
Giet, R. & Prigent, C. Aurora/Ipl1p-related kinases, a new oncogenic family of mitotic serine-threonine kinases. J. Cell. Sci. 112, 3591–3601 (1999).
Zou, H., McGarry, T. J., Bernal, T. & Kirschner, M. W. Identification of a vertebrate sister-chromatid separation inhibitor involved in transformation and tumorigenesis. Science 285, 418–422 (1999).
Tao, W. et al. Human homologue of the Drosophila melanogaster lats tumour suppressor modulates CDC2 activity. Nature Genet. 21, 177–181 (1999).
St John, M. A. R. et al. Mice deficient of Lats1 develop soft-tissue sarcomas, ovarian tumours and pituitary dysfunction. Nature Genet. 21, 182–186 (1999).References 107 and 108 provide the first link between CDK1 deregulation and tumour development. These reports raise important questions regarding a possible role of CDK1 in cell transformation.
Vesely, J. et al. Inhibition of cyclin-dependent kinases by purine analogues. Eur. J. Biochem. 224, 771–786 (1994).
De Azevedo, W. F., Leclerc, S., Meijer, L., Strand, M. & Kim, S. H. Inhibition of cyclin-dependent kinases by purine analogues: crystal structure of human cdk2 complexed with roscovitine. Eur. J. Biochem. 243, 518–526 (1997).
Arris, C. E. et al. Identification of novel purine and pyrimidine cyclin-dependent kinase inhibitors with distinct molecular interactions and tumor cell growth inhibition profiles. J. Med. Chem. 43, 2797–2804 (2000).
Zaharevitz, D. W. et al. Discovery and initial characterization of the paullones, a novel class of small-molecule inhibitors of cyclin-dependent kinases. Cancer Res. 59, 2566–2569 (1999).
Kitagawa et al. A cyclin-dependent kinase inhibitor, butyrolactone I, inhibits phosphorylation of RB protein and cell cycle progression. Oncogene 9, 2549–2557 (1994).
Meijer, L. et al. Inhibition of cyclin-dependent kinases, GSK-3β and CK1 by hymenialdisine, a marine sponge constituent. Chem. Biol. 7, 51–63 (1999).
Lane, M. E. et al. A novel cdk2-inhibitor, SU9516, induces apoptosis in colon carcinoma cells. Cancer Res. 61, 6170–6177 (2001).
Soni, R. et al. Selective in vivo and in vitro effects of a small molecule inhibitor of cyclin-dependent kinase 4. J. Natl Cancer Inst. 93, 436–446 (2001).
Fry, D. W. et al. Cell cycle and biochemical effects of PD 0183812. A potent inhibitor of the cyclin D-dependent kinases CDK4 and CDK6. J. Biol. Chem. 276, 16617–16623 (2001).
Soni, R. et al. Inhibition of cyclin–dependent kinase 4 (Cdk4) by fascaplysin, a marine natural product. Biochem. Biophys. Res. Commun. 275, 877–884 (2000).
Author information
Authors and Affiliations
Supplementary information
Related links
Related links
DATABASES
FURTHER INFORMATION
BioMedNet Mouse Knockout & Mutation Database
Glossary
- METAZOANS
-
The kingdom Animalia (animals) that consists of roughly 35 phyla of multicellular organisms.
- T LOOP
-
A short peptide sequence — located between subdomains VII and VIII of protein kinases — that folds as a loop. Phosphorylation might alter its conformation and, hence, the catalytic activity of the protein kinases.
- RAS/RAF/MAPK PATHWAY
-
Signal-transduction pathway that is crucial for the integration of mitogenic signals mediated by a variety of growth factors. Activation of this pathway is involved in many cellular processes, including cell-cycle progression.
- SCF UBIQUITIN LIGASE
-
Multiprotein complex that ligates ubiquitin to proteins that will be degraded by the proteasome. SCF complexes consist of three core subunits (SKP1, CUL1 and RBX1) coupled to one of the several F-box proteins that recognize the protein to be ubiquitylated.
- RAS/PI3K/AKT PATHWAY
-
Signal-transduction pathway that is involved in many cellular processes, such as cell proliferation and apoptosis. Several members of this pathway have oncogenic or tumour-suppressor activities (for example, RAS, PI3K and PTEN).
- DOMINANT-NEGATIVE MUTANT
-
A non-functional mutant protein that competes with the normal, non-mutated protein, blocking its activity.
- PROTEASOME
-
A 26S multiprotein complex that catalyzes the breakdown of polyubiquitylated proteins.
- F-BOX PROTEIN
-
Component of the machinery for the ubiquitin-dependent degradation of proteins. F-box proteins recognize specific substrates and present them to the SCF complex and the proteasome machinery.
- ORGANOMEGALY
-
A phenotype characterized by organs of abnormally large size.
- HAPLO-INSUFFICIENCY
-
A phenotype that arises in diploid organisms due to the loss of only one allele.
- PAN-CDK INHIBITORS
-
CDK inhibitors that do not discriminate between different CDKs. These drugs can frequently inhibit other protein kinases.
Rights and permissions
About this article
Cite this article
Malumbres, M., Barbacid, M. To cycle or not to cycle: a critical decision in cancer. Nat Rev Cancer 1, 222–231 (2001). https://doi.org/10.1038/35106065
Issue Date:
DOI: https://doi.org/10.1038/35106065
This article is cited by
-
Uncovering Cell Cycle Dysregulations and Associated Mechanisms in Cancer and Neurodegenerative Disorders: A Glimpse of Hope for Repurposed Drugs
Molecular Neurobiology (2024)
-
Differential expression of the circadian clock network correlates with tumour progression in gliomas
BMC Medical Genomics (2023)
-
Cyclers’ kinases in cell division: from molecules to cancer therapy
Cell Death & Differentiation (2023)
-
PSMA-617 inhibits proliferation and potentiates the 177Lu-PSMA-617-induced death of human prostate cancer cells
Naunyn-Schmiedeberg's Archives of Pharmacology (2023)
-
Cyclins and cyclin-dependent kinases: from biology to tumorigenesis and therapeutic opportunities
Journal of Cancer Research and Clinical Oncology (2023)