Abstract
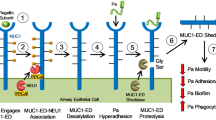
Cell-surface heparan sulphate proteoglycans (HSPGs) are ubiquitous and abundant receptors/co-receptors of extracellular ligands1,2, including many microbes3,4,5,6,7,8,9,10. Their role in microbial infections is poorly defined, however, because no cell-surface HSPG has been clearly connected to the pathogenesis of a particular microbe. We have previously shown that Pseudomonas aeruginosa, through its virulence factor LasA, enhances the in vitro shedding of syndecan-1—the predominant cell-surface HSPG of epithelia11. Here we show that shedding of syndecan-1 is also activated by P. aeruginosa in vivo, and that the resulting syndecan-1 ectodomains enhance bacterial virulence in newborn mice. Newborn mice deficient in syndecan-1 resist P. aeruginosa lung infection but become susceptible when given purified syndecan-1 ectodomains or heparin, but not when given ectodomain core protein, indicating that the ectodomain's heparan sulphate chains are the effectors. In wild-type newborn mice, inhibition of syndecan-1 shedding or inactivation of the shed ectodomain's heparan sulphate chains prevents lung infection. Our findings uncover a pathogenetic mechanism in which a host response to tissue injury—syndecan-1 shedding—is exploited to enhance microbial virulence apparently by modulating host defences.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Park, P. W., Reizes, O. & Bernfield, M. Cell surface heparan sulfate proteoglycans: selective regulators of ligand-receptor encounters. J. Biol. Chem. 275, 29923–29926 (2000).
Bernfield, M. et al. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68, 729–777 (1999).
Shieh, M. T., WuDunn, D., Montgomery, R. I., Esko, J. D. & Spear, P. G. Cell surface receptors for herpes simplex virus are heparan sulfate proteoglycans. J. Cell Biol. 116, 1273–1281 (1992).
Shukla, D. et al. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99, 13–22 (1999).
Chen, Y. et al. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat. Med. 3, 866–871 (1997).
Pancake, S. J., Holt, G. D., Mellouk, S. & Hoffman, S. L. Malaria sporozoites and circumsporozoite proteins bind specifically to sulfated glycoconjugates. J. Cell Biol. 117, 1351–1357 (1992).
Ortega-Barria, E. & Pereira, M. E. A novel T. cruzi heparin-binding protein promotes fibroblast adhesion and penetration of engineered bacteria and trypanosomes into mammalian cells. Cell 67, 411–421 (1991).
Menozzi, F. D. et al. Identification of a heparin-binding hemagglutinin present in Mycobacteria. J. Exp. Med. 184, 993–1001 (1996).
Grassmé, H. et al. Acidic sphingomyelinase mediates entry of N. gonorrhoeae into nonphagocytic cells. Cell 91, 605–615 (1997).
Rostand, K. S. & Esko, J. D. Microbial adherence to and invasion through proteoglycans. Infect. Immun. 65, 1–8 (1997).
Park, P. W. et al. Syndecan-1 shedding is enhanced by LasA, a secreted virulence factor of Pseudomonas aeruginosa. J. Biol. Chem. 275, 3057–3062 (2000).
Pier, G. B. et al. Role of mutant CFTR in hypersusceptibility of cystic fibrosis patients to lung infections. Science 271, 64–67 (1996).
Tang, H., Kays, M. & Prince, A. Role of Pseudomonas aeruginosa pili in acute pulmonary infection. Infect. Immun. 63, 1278–1285 (1995).
Allewelt, M., Coleman, F. T., Grout, M., Priebe, G. P. & Pier, G. B. Acquisition of expression of the Pseudomonas aeruginosa ExoU cytotoxin leads to increased bacterial virulence in a murine model of acute pneumonia and systemic spread. Infect. Immun. 68, 3998–4004 (2000).
Alexander, C. M. et al. Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nature Genet. 25, 329–332 (2000).
Rostand, K. S. & Esko, J. D. Cholesterol and cholesterol esters: host receptors for Pseudomonas aeruginosa adherence. J. Biol. Chem. 268, 24053–24059 (1993).
Kato, M. et al. Physiological degradation converts the soluble syndecan-1 ectodomain from an inhibitor to a potent activator of FGF-2. Nature Med. 4, 691–697 (1998).
Subramanian, S. V., Fitzgerald, M. L. & Bernfield, M. Regulated shedding of syndecan-1 and -4 ectodomains by thrombin and growth factor activation. J. Biol. Chem. 272, 14713–14720 (1997).
Woods, D. E., Cryz, S. J., Friedman, R. L. & Iglewski, B. H. Contribution of toxin A and elastase to virulence of Pseudomonas aeruginosa in chronic lung infections of rats. Infect. Immun. 36, 1223–1228 (1982).
Blackwood, L. L., Stone, R. M., Iglewski, B. H. & Pennington, J. E. Evaluation of Pseudomonas aeruginosa exotoxin A and elastase as virulence factors in acute lung infection. Infect. Immun. 39, 198–201 (1983).
Huttner, K. M. & Bevins, C. L. Antimicrobial peptides as mediators of epithelial host defense. Pediatr. Res. 45, 785–794 (1999).
Bals, R., Weiner, D. J. & Wilson, J. M. The innate immune system in cystic fibrosis lung disease. J. Clin. Invest. 103, 303–307 (1999).
Kainulainen, V., Wang, H., Schick, C. & Bernfield, M. Syndecans, heparan sulfate proteoglycans, maintain the proteolytic balance of acute wound fluids. J. Biol. Chem. 273, 11563–11569 (1998).
Belaaouaj, A. et al. Mice lacking neutrophil elastase reveal impaired host defense against Gram negative bacterial sepsis. Nature Med. 4, 615–618 (1998).
LeVine, A. M. et al. Surfactant protein-A-deficient mice are susceptible to Pseudomonas aeruginosa infection. Am. J. Respir. Cell Mol. Biol. 19, 700–708 (1998).
Crouch, E. C. Collectins and pulmonary host defense. Am. J. Respir. Cell Mol. Biol. 19, 177–201 (1998).
Kuschert, G. S. V. V. et al. Glycosaminoglycans interact selectively with chemokines and modulate receptor binding and cellular responses. Biochemistry 38, 12959–12968 (1999).
Pearson, J. P., Feldman, M., Iglewski, B. H. & Prince, A. Pseudomonas aeruginosa cell-to-cell signaling is required for virulence in a model of acute pulmonary infections. Infect. Immun. 68, 4331–4334 (2000).
Schmidtchen, A., Frick, I. & Bjorck, L. Dermatan sulphate is released by proteinases of common pathogenic bacteria and inactivates antibacterial alpha-defensin. Mol. Microbiol. 39, 708–713 (2001).
Acknowledgements
We thank A. Drummond and P. Kincade for the peptide hydroxamate (BB1101) and the Ky 8.2 monoclonal antibody, respectively. This work was supported by the Parker B. Francis Foundation (P.W.P.) and the NIH (G.B.P. and M.B.).
Author information
Authors and Affiliations
Corresponding author
Supplementary information

Figure 1.
(GIF 11.3 kb)
For 35S-labeled syndecan-1, 9050 cpm (2 ng) was incubated with 5x109 cfus of P. aeruginosa strains (PAO1, BL2, or 6077) in 300 µl of TSB with or without heparin or HS-free ectodomain core protein (both 40 µg) at room temperature. After 1 h, the mixtures were spun down, washed once with buffer, and the amount of radioactivity associated with P. aeruginosa was quantified by scintillationcounting. The binding assay using 125I-labeled syndecan-1 followed the same protocol except that 105,500 cpm (67 ng) of the labeled syndecan-1 was incubated with 1x1010 cfus of P. aeruginosa strain BL2, and binding was quantified by counting in a g counter.P. aeruginosa strains did not bind syndecan-1 labeled by either 35S or 125I significantly above background levels (23 cpm & 900 cpm, respectively). Results are shown as mean±SD from triplicate measurements.

Figure 2.
(GIF 13.1 kb)
For the pre-treatment experiments, P. aeruginosa strain PAO1 was incubated with 5 ng/µl of heparin in 50 µl of TSB for 30 min at room temperature, then washed (2x) with 1 ml of TSB. The inoculum for the non-treated control groups was 2.1x109 cfus/7 µl, whereasthose for the pre-treated bacteria/wild type synd-1+/+ and pre-treated bacteria/synd-1-/- mice were 1.4x109 cfus/7 µl and 1.9x109 cfus/7 µl, respectively. The extent of infection was assessed by quantifying lung (pneumonia) and spleen (bacteremia)colonization, and by observing mortality as described in our manuscript.

Figure 3.
(GIF 7.09 kb)
Early log growth phase bacteria (E. coli, JM101) were incubated in 500 µl of TSB with or without the neutrophil-derived antimicrobial peptide bactenecin-7 (10 µg), shed syndecan-1 ectodomain (5 µg), or heparin (5 µg) as indicated at 37°C with agitation. After 8 h, bacterial viability was measured by the tetrazolium salt conversion assay, in which only live cells will convert the tetrazolium salt (MTT) to insoluble formazan (purple). Formazan is solubilized by DMSO and the samples are read at OD550nm. Results shown are mean±SD from triplicate measurements.

Figure 4.
(GIF 5.05 kb)
Bactenecin-7 was radioiodinated by the IODOGEN method, and approximately 500 ng (177,000 cpm) of the iodinated antimicrobial was incubated with 8x109 cfus of bacteria (E. coli, JM101) with or without heparin or chondroitin sulfate C (both 20 µg) in 300 µl of TSB. After 30 min, cells were washed once with TSB and radioactivity associated with the cell pellet was quantified in a g counter.Background was determined in the absence of bacteria and glycosaminoglycans. Data shown are mean±SD of triplicate measurements.

Figure 5.
(GIF 5.4 kb)
Early log growth phase bacteria (E. coli, JM101) were incubated in 500 µl of TSB with bactenecin-7, PR-39, or cecropin B (all at 1.5 mM) with or without heparin (5 µg). After 3 h, bacterial viability was measured by the tetrazolium salt conversion assay as described in Appendix 3. Results shown are mean±SD of triplicate measurements.

Figure 6.
(GIF 9.6 kb)
Mice were inoculated intraperitoneally with P. aeruginosa strain PAO1 in 100 µl of TSB. After 20 h, lungs were harvested, strained through a stainless steel mesh, incubated in TSB containing 0.1% (v/v) Triton X-100, diluted in TSB, and plated out on TSB agar plates. Lung colonization (bacteremia) values shown are mean±SE, and mortality values are shown in parentheses above the bar graphs.
Rights and permissions
About this article
Cite this article
Park, P., Pier, G., Hinkes, M. et al. Exploitation of syndecan-1 shedding by Pseudomonas aeruginosa enhances virulence. Nature 411, 98–102 (2001). https://doi.org/10.1038/35075100
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35075100
This article is cited by
-
Chemical editing of proteoglycan architecture
Nature Chemical Biology (2022)
-
The heparan sulfate proteoglycan Syndecan-1 influences local bone cell communication via the RANKL/OPG axis
Scientific Reports (2020)
-
Soluble Syndecan-1: A Novel Biomarker of Small Bowel Mucosal Damage in Children with Celiac Disease
Digestive Diseases and Sciences (2017)
-
Endothelial Glycocalyx as Biomarker for Cardiovascular Diseases: Mechanistic and Clinical Implications
Current Heart Failure Reports (2017)
-
Syndecan-1 (CD138) deficiency increases Staphylococcus aureus infection but has no effect on pathology in a mouse model of peritoneal dialysis
Journal of Biomedical Science (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.