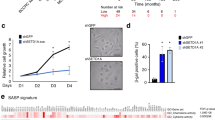
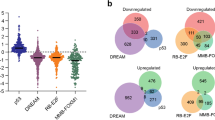
Abstract
The p16INK4a cyclin-dependent kinase inhibitor1 is implicated in replicative senescence, the state of permanent growth arrest provoked by cumulative cell divisions or as a response to constitutive Ras–Raf–MEK signalling in somatic cells2,3,4,5,6,7,8. Some contribution to senescence presumably underlies the importance of p16INK4a as a tumour suppressor9 but the mechanisms regulating its expression in these different contexts remain unknown. Here we demonstrate a role for the Ets1 and Ets2 transcription factors10 based on their ability to activate the p16INK4a promoter through an ETS-binding site and their patterns of expression during the lifespan of human diploid fibroblasts. The induction of p16INK4a by Ets2, which is abundant in young human diploid fibroblasts, is potentiated by signalling through the Ras–Raf–MEK kinase cascade and inhibited by a direct interaction with the helix–loop–helix protein Id1 (ref. 11). In senescent cells, where the Ets2 levels and MEK signalling decline, the marked increase in p16INK4a expression is consistent with the reciprocal reduction of Id1 and accumulation of Ets1.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Serrano, M., Hannon, G. J. & Beach, D. A new regulatory motif in cell cycle control causing specific inhibition of cyclinD/CDK4. Nature 366, 704–707 (1993).
Alcorta, D. A. et al. Involvement of the cyclin-dependent kinase inhibitor p16 (INK4a) in replicative senescence. Proc. Natl Acad. Sci. USA 93, 13742–13747 (1996).
Hara, E. et al. Regulation of p16CDKN2 expression and its implications for cell immortalization and senescence. Mol. Cell. Biol. 16, 859–867 (1996).
Loughran, O. et al. Association of CDKN2A/p16INK4a with human head and neck keratinocyte replicative senescence: relationship of dysfunction to immortality and neoplasia. Oncogene 13, 561–568 (1996).
Reznikoff, C. A. et al. Elevated p16 at senescence and loss of p16 at immortalization in human papillomavirus 16 E6, but not E7, transformed human uroepithelial cells. Cancer Res. 56, 2886–2890 (1996).
Serrano, M., Lin, A. W., McCurrach, M. E., Beach, D. & Lowe, S. W. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell 88, 593–602 (1997).
Lin, A. W. et al. Premature senescence involving p53 and p16 is activated in response to constitutive MEK/MAPK mitogenic signaling. Genes Dev. 12, 3008–3019 (1998).
Zhu, J., Woods, D., McMahon, M. & Bishop, J. M. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 12, 2997–3007 (1998).
Ruas, M. & Peters, G. The p16INK4a/CDKN2A tumor suppressor and its relatives. Biochim. Biophys. Acta 1378, 115–117 (1998).
Graves, B. J. & Petersen, J. M. Specificity within the ets family of transcription factors. Adv. Cancer Res. 75, 1–55 (1998).
Benezra, R., Davis, R. L., Lockshon, D., Turner, D. L. & Weintraub, H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell 61, 49–59 (1990).
Sherr, C. J. & Roberts, J. M. CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev. 13, 1501–1512 (1999).
Hayflick, L. & Moorhead, P. S. The serial cultivation of human diploid cell strains. Exp. Cell Res. 25, 585–621 (1961).
Lundberg, A. S., Hahn, W. C., Gupta, P. & Weinberg, R. A. Genes involved in senescence and immortalization. Curr. Opin. Cell Biol. 12, 705–709 (2000).
Harley, C. B., Futcher, A. B. & Greider, C. W. Telomeres shorten during ageing of human fibroblasts. Nature 345, 458–460 (1990).
Sedivy, J. M. Can ends justify the means?: telomeres and the mechanisms of replicative senescence and immortalization in mammalian cells. Proc. Natl Acad. Sci. USA 95, 9078–9081 (1998).
McConnell, B. B., Starborg, M., Brookes, S. & Peters, G. Inhibitors of cyclin-dependent kinases induce features of replicative senescence in early passage human diploid fibroblasts. Curr. Biol. 8, 351–354 (1998).
Foos, G., Garcia-Ramirez, J. J., Galang, C. K. & Hauser, C. A. Elevated expression of Ets2 or distinct portions of Ets2 can reverse ras-mediated cellular transformation. J. Biol. Chem. 273, 18871–18880 (1998).
Dimri, G. P. et al. A novel biomarker identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl Acad. Sci. USA 92, 9363–9367 (1995).
Yates, P. R., Atherton, G. T., Deed, R. W., Norton, J. D. & Sharrocks, A. D. Id helix-loop-helix proteins inhibit nucleoprotein complex formation by the TCF ETS-domain transcription factors. EMBO J. 18, 968–976 (1999).
Massari, M. E. & Murre, C. Helix-loop-helix proteins: regulators of transcription in eucaryotic organisms. Mol. Cell. Biol. 20, 429–440 (2000).
Hara, E. et al. Id-related genes encoding helix-loop-helix proteins are required for G1 progression and are repressed in senescent human fibroblasts. J. Biol. Chem. 269, 2139–2145 (1994).
Lyden, D. et al. Id1 and Id3 are required for neurogenesis, angiogenesis and vascularization of tumour xenografts. Nature 401, 670–677 (1999).
Hara, E. et al. The helix-loop-helix protein Id-1 and a retinoblastoma protein binding mutant of SV40 T antigen synergize to reactivate DNA synthesis in senescent human fibroblasts. Dev. Genet. 18, 161–172 (1996).
Alani, R. M. et al. Immortalization of primary human keratinocytes by the helix-loop-helix protein, Id-1. Proc. Natl Acad. Sci. USA 96, 9637–9641 (1999).
Jacobs, J. J. L., Kieboom, K., Marino, S., DePinho, R. A. & van Lohuizen, M. The oncogene and Polycomb-group gene bmi-1 regulates cell proliferation and senescence through the ink4a locus. Nature 397, 164–168 (1999).
Takahashi, Y., Rayman, J. B. & Dynlacht, B. D. Analysis of promoter binding by the E2F and pRB families in vivo: distinct E2F proteins mediate activation and repression. Genes Dev. 14, 804–816 (2000).
Sugimoto, M. et al. Regulation of CDK4 activity by a novel CDK4-binding protein, p34SEI-1. Genes Dev. 13, 3027–3033 (1999).
Acknowledgements
We thank C. A. Hauser, K. E. Boulukos, N. G. Ahn, Y. de Launoit, Y. Nagamine and J. Ghysdael for providing useful materials. We are also grateful to N. Jones, R. Treisman, J. Campisi, I. Palmero and M. Serrano for helpful discussions, and to M. Hughes and J. Barry for help in FACS. We also thank T. Tanaka for his useful suggestion in ChIP assay and S. Bagley and T. D. Allen for help in using the microscope. This work was supported by the Cancer Research Campaign, Imperial Cancer Research Fund and Nihon Schering KK.
Author information
Authors and Affiliations
Corresponding author
Supplementary information
Rights and permissions
About this article
Cite this article
Ohtani, N., Zebedee, Z., Huot, T. et al. Opposing effects of Ets and Id proteins on p16INK4a expression during cellular senescence. Nature 409, 1067–1070 (2001). https://doi.org/10.1038/35059131
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35059131
This article is cited by
-
ID2 promotes tumor progression and metastasis in thyroid cancer
Endocrine (2024)
-
Effect of aging on the formation and growth of colonic epithelial organoids by changes in cell cycle arrest through TGF-β-Smad3 signaling
Inflammation and Regeneration (2023)
-
ID1 expressing macrophages support cancer cell stemness and limit CD8+ T cell infiltration in colorectal cancer
Nature Communications (2023)
-
The roles and mechanisms of senescence-associated secretory phenotype (SASP): can it be controlled by senolysis?
Inflammation and Regeneration (2022)
-
The landscape of human tissue and cell type specific expression and co-regulation of senescence genes
Molecular Neurodegeneration (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.