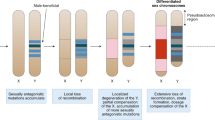
Abstract
The molecular mechanisms that control sexual dimorphism are very different in distantly related animals. Did sex determination arise several times with different regulatory mechanisms, or is it an ancient process with little surviving evidence of ancestral genes? The recent identification of related sexual regulators in different phyla indicates that some aspects of sexual regulation might be ancient. Studies of sex-determining mechanisms are beginning to reveal how sexual dimorphism arises and evolves.
Key Points
-
Sex-determining pathways in different species are initiated by a surprising variety of genetic and environmental signals.
-
Sex chromosomes can evolve quickly and generally are not conserved at the molecular level, between distant species.
-
Experimental manipulations of Caenorhabditis elegans show how sex-determination mechanisms can be completely transformed by simple mutations in regulatory genes.
-
Comparisons of nematodes with insects indicate that sexual regulators that function upstream in the developmental hierarchy might evolve very rapidly, but that downstream genes are more highly conserved.
-
Flies, worms and mammals regulate the various aspects of sexual development with genes that contain the DM-domain DNA-binding motif.
-
The conservation of downstream sex-determining genes and the divergence of those upstream fits theoretical predictions.
-
Sexual regulation must be integrated with spatial and temporal regulation. The control of sexual dimorphism in the posterior of flies and worms illustrates how this can occur.
-
Short-range transcriptional repression seems to generate sexual dimorphism in worms, and might be a general mechanism in other species.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Barton, N. H. & Charlesworth, B. Why sex and recombination? Science 281, 1986–1989 (1998).
Welch, D. M. & Meselson, M. Evidence for the evolution of bdelloid rotifers without sexual reproduction or genetic exchange. Science 288, 1211–1215 ( 2000).
Marin, I. & Baker, B. S. The evolutionary dynamics of sex determination. Science 281, 1990– 1994 (1998).
Sinclair, A. H. et al. A gene from the human sex-determining region encodes a protein with homology to a conserved DNA-binding motif. Nature 346, 240–244 (1990).
Cline, T. W. & Meyer, B. J. Vive la difference: males vs females in flies vs worms. Annu. Rev. Genet. 30, 637–702 (1996).
Fredga, K. in The Differences Between the Sexes (eds Short, R. V. & Balaban, E.) 419–431 (Cambridge Univ. Press, Cambridge, 1994).
Schutt, C. & Nothinger, R. Structure, function and evolution of sex-determining systems in dipteran insects. Development 127, 667–677 (2000).
Graves, J. A. Mammals that break the rules: genetics of marsupials and monotremes. Annu. Rev. Genet. 30, 233–260 (1996).
Charlesworth, B. The evolution of chromosomal sex determination and dosage compensation. Curr. Biol. 6, 149–162 ( 1996).
Hodgkin, J. Genetic sex determination mechanisms and evolution. Bioessays 14, 253–261 (1992).
Bull, J. J. Evolution of Sex Determining Mechanisms (Benjamin/Cummings, Menlo Park, California, 1983).
Stevanovic, M., Lovell-Badge, R., Collignon, J. & Goodfellow, P. N. SOX3 is an X-linked gene related to SRY. Hum. Mol. Genet. 2, 2013–2018 ( 1993).
Foster, J. W. & Graves, J. A. An SRY-related sequence on the marsupial X chromosome: implications for the evolution of the mammalian testis-determining gene. Proc. Natl Acad. Sci. USA 91, 1927–1931 (1994).
Lahn, B. T. & Page, D. C. Four evolutionary strata on the human X chromosome. Science 286, 964– 967 (1999).
Marin, I., Siegal, M. L. & Baker, B. S. The evolution of dosage-compensation mechanisms. Bioessays 22, 1106–1114 (2000).
Marin, I., Franke, A., Bashaw, G. J. & Baker, B. S. The dosage compensation system of Drosophila is co-opted by newly evolved X chromosomes. Nature 383, 160– 163 (1996).
Bopp, D., Calhoun, G., Horabin, J. I., Samuels, M. & Schedl, P. Sex-specific control of Sex-lethal is a conserved mechanism for sex determination in the genus Drosophila . Development 122, 971– 982 (1996).
O'Neil, M. T. & Belote, J. M. Interspecific comparison of the transformer gene of Drosophila reveals an unusually high degree of evolutionary divergence. Genetics 131, 113–128 (1992).
Chandler, D. et al. Evolutionary conservation of regulatory strategies for the sex determination factor transformer-2. Mol. Cell. Biol. 17, 2908–2919 ( 1997).
Muller-Holtkamp, F. The Sex-lethal homologue in Chrysomya rufifacies is highly conserved in sequence and exon–intron organization. J. Mol. Evol. 41, 467–477 (1995).
Sievert, V., Kuhn, S. & Traut, W. Expression of the sex determining cascade genes Sex-lethal and doublesex in the phorid fly Megaselia scalaris. Genome 40, 211–214 ( 1997).
Meise, M. et al. Sex-lethal, the master sex-determining gene in Drosophila , is not sex-specifically regulated in Musca domestica. Development 125, 1487–1494 (1998).
Dauwalder, B., Amaya-Manzanares, F. & Mattox, W. A human homologue of the Drosophila sex determination factor transformer-2 has conserved splicing regulatory functions. Proc. Natl Acad. Sci. USA 93, 9004– 9009 (1996).
Beil, B., Screaton, G. & Stamm, S. Molecular cloning of htra2-β-1 and htra2-β-2 , two human homologues of tra-2 generated by alternative splicing . DNA Cell Biol. 16, 679– 690 (1997).
Tacke, R., Tohyama, M., Ogawa, S. & Manley, J. L. Human TRA2 proteins are sequence-specific activators of pre-mRNA splicing. Cell 93, 139–148 (1998).
Shearman, D. C. & Frommer, M. The Bactrocera tryoni homologue of the Drosophila melanogaster sex-determination gene doublesex. Insect Mol. Biol. 7, 355–366 (1998).
Shimada, T. & Ohbayashi, F. Bombyx mori Bmdsx mRNA for BmDSX-M, complete cds, male specific (AB48544); and mRNA for BmDSX–F, complete cds, female specific (AB048543). GenBank ( 2000).[style?]
de Bono, M. & Hodgkin, J. Evolution of sex determination in Caenorhabditis: unusually high divergence of tra-1 and its functional consequences. Genetics 144, 587– 595 (1996).
Kuwabara, P. E. Interspecies comparison reveals evolution of control regions in the nematode sex-determining gene tra-2. Genetics 144, 597–607 (1996).
Kuwabara, P. E. & Shah, S. Cloning by synteny: identifying C. briggsae homologues of C. elegans genes. Nucleic Acids Res. 22, 4414–4418 (1994).
Streit, A. et al. Homologs of the Caenorhabditis elegans masculinizing gene her-1 in C. briggsae and the filarial parasite Brugia malayi. Genetics 152, 1573– 1584 (1999).
Ventura-Holman, T., Seldin, M. F., Li, W. & Maher, J. F. The murine fem1 gene family: homologs of the Caenorhabditis elegans sex-determination protein FEM-1. Genomics 54, 221– 230 (1998).
Raymond, C. S. et al. Evidence for evolutionary conservation of sex-determining genes. Nature 391, 691– 695 (1998).
Erdman, S. E. & Burtis, K. C. The Drosophila doublesex proteins share a novel zinc finger related DNA binding domain. EMBO J. 12, 527–535 ( 1993).
Zhu, L. et al. Sexual dimorphism in diverse metazoans is regulated by a novel class of intertwined zinc fingers. Genes Dev. 14, 1750–1764 (2000).
Baker, B. S. & Ridge, K. Sex and the single cell: on the action of major loci affecting sex determination in Drosophila melanogaster. Genetics 94, 383–423 ( 1980).
Coschigano, K. T. & Wensink, P. C. Sex-specific transcriptional regulation by male and female doublesex proteins of Drosophila . Genes Dev. 7, 42– 54 (1993).
An, W. & Wensink, P. C. Integrating sex- and tissue-specific regulation within a single Drosophila enhancer. Genes Dev. 9, 256–266 ( 1995).Together with reference 37 , this paper shows that the two sex-specific isoforms of Dsx bind the same DNA site and have opposite effects on the transcription of the Yp1 yolk-protein gene.
Yi, W. & Zarkower, D. Similarity of DNA binding and transcriptional regulation by Caenorhabditis elegans MAB-3 and Drosophila melanogaster DSX suggests conservation of sex determining mechanisms. Development 126, 873–881 (1999).
Shen, M. M. & Hodgkin, J. mab-3, a gene required for sex-specific yolk protein expression and a male-specific lineage in C. elegans. Cell 54, 1019– 1031 (1988).
Yi, W., Ross, J. M. & Zarkower, D. mab-3 is a direct tra-1 target gene that regulates diverse aspects of C. elegans male sexual behavior. Development 127, 4469–4480 ( 2000).
Nef, S. & Parada, L. F. Hormones in male sexual development . Genes Dev. 14, 3075–3086 (2000).
Jost, A. Recherches sur le differentiation sexuelle de l'embryo de lapin. Arch. Anat. Microsc. Morphol. Exp. 36, 117– 121 (1947).
Jost, A. Studies on sex differentiation in mammals. Rec. Prog. Horm. Res. 8, 379–418 ( 1953).
Zimmermann, S. et al. Targeted disruption of the Insl3 gene causes bilateral cryptorchidism. Mol. Endocrinol. 13, 681 –691 (1999).
Nef, S. & Parada, L. F. Cryptorchidism in mice mutant for Insl3. Nature Genet. 22, 295– 299 (1999).
Nachtigal, M. W. et al. Wilms' tumor 1 and Dax-1 modulate the orphan nuclear receptor SF-1 in sex-specific gene expression. Cell 93, 445–454 (1998).Shows that Sf1 and Wt1 synergistically activate Müllerian inhibiting substance and that Dax1 antagonizes this activation in transfected cells. Provides evidence for physical interaction between Sf1 and Wt1 and between Sf1 and Dax1.
Swain, A., Narvaez, V., Burgoyne, P., Camerino, G. & Lovell-Badge, R. Dax1 antagonizes Sry action in mammalian sex determination. Nature 391, 761–767 (1998).
Vainio, S., Heikkila, M., Kispert, A., Chin, N. & McMahon, A. P. Female development in mammals is regulated by Wnt-4 signalling. Nature 397, 405–409 (1999).
McElreavey, K., Vilain, E., Abbas, N., Herskowitz, I. & Fellous, M. A regulatory cascade hypothesis for mammalian sex determination: SRY represses a negative regulator of male development. Proc. Natl Acad. Sci. USA 90, 3368–3372 (1993).
Swain, A. & Lovell-Badge, R. Mammalian sex determination: a molecular drama. Genes Dev. 13, 755– 767 (1999).
Capel, B. The battle of the sexes. Mech. Dev. 92, 89–103 (2000).
Gubbay, J. et al. A gene mapping to the sex-determining region of the mouse Y chromosome is a member of a novel family of embryonically expressed genes . Nature 346, 245–250 (1990).
Koopman, P., Gubbay, J., Vivian, N., Goodfellow, P. & Lovell-Badge. R. Male development of chromosomally female mice transgenic for Sry. Nature 351, 117– 121 (1991).Provides formal proof that Sry is sufficient to induce male development in an XX mammal.
Tucker, P. K. & Lundrigan, B. L. Rapid evolution of the sex determining locus in Old World mice and rats. Nature 364, 715–717 (1993).
Whitfield, L. S., Lovell-Badge, R. & Goodfellow, P. N. Rapid sequence evolution of the mammalian sex-determining gene SRY. Nature 364, 713– 715 (1993).
Kent, J., Wheatley, S. C., Andrews, J. E., Sinclair, A. H. & Koopman, P. A male-specific role for SOX9 in vertebrate sex determination. Development 122, 2813–2822 (1996).
Morais da Silva, S. et al. Sox9 expression during gonadal development implies a conserved role for the gene in testis differentiation in mammals and birds . Nature Genet. 14, 62– 68 (1996).
Oreal, E. et al. Early expression of AMH in chicken embryonic gonads precedes testicular SOX9 expression. Dev. Dyn. 212, 522–532 (1998).
Spotila, L. D., Spotila, J. R. & Hall, S. E. Sequence and expression analysis of WT1 and Sox9 in the red-eared slider turtle, Trachemys scripta. J. Exp. Zool. 281, 417–427 (1998).
Moreno-Mendoza, N., Harley, V. R. & Merchant-Larios, H. Differential expression of SOX9 in gonads of the sea turtle Lepidochelys olivacea at male- or female-promoting temperatures . J. Exp. Zool. 284, 705– 710 (1999).
Western, P. S., Harry, J. L., Graves, J. A. & Sinclair, A. H. Temperature-dependent sex determination: upregulation of SOX9 expression after commitment to male development. Dev. Dyn. 214 , 171–177 (1999).
Foster, J. W. et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature 372 , 525–530 (1994).
Wagner, T. et al. Autosomal sex reversal and campomelic dysplasia are caused by mutations in and around the SRY-related gene SOX9. Cell 79, 1111–1120 ( 1994).
Bishop, C. E. et al. A transgenic insertion upstream of Sox9 is associated with dominant XX sex reversal in the mouse. Nature Genet. 26, 490–494 (2000). A fortuitous transgene insertion far upstream of the Sox9 gene causes constitutive expression of Sox9 in XX embryos and results in fully male development, showing clearly the importance of Sox9 in mammals. The insertion might interfere with a negative regulatory element, consistent with the regulatory hypothesis described in reference 50.
Raymond, C. S., Kettlewell, J. R., Hirsch, B., Bardwell, V. J. & Zarkower, D. Expression of Dmrt1 in the genital ridge of mouse and chicken embryos suggests a role in vertebrate sexual development. Dev. Biol. 215, 208– 220 (1999).
De Grandi, A. et al. The expression pattern of a mouse doublesex-related gene is consistent with a role in gonadal differentiation. Mech. Dev. 90, 323–326 ( 2000).
Smith, C. A., McClive, P. J., Western, P. S., Reed, K. J. & Sinclair, A. H. Conservation of a sex-determining gene. Nature 402, 601–602 (1999).
Moniot, B., Berta, P., Scherer, G., Sudbeck, P. & Poulat, F. Male specific expression suggests role of DMRT1 in human sex determination. Mech. Dev. 91, 323–325 (2000).
Kettlewell, J. R., Raymond, C. S. & Zarkower, D. Temperature-dependent expression of turtle Dmrt1 prior to sexual differentiation. Genesis 26, 174–178 (2000).
Marchand, O. et al. DMRT1 expression during gonadal differentiation and spermatogenesis in the rainbow trout, Oncorhynchus mykiss. Biochim. Biophys. Acta 1493, 180–187 (2000).
Raymond, C. S. et al. A region of human chromosome 9p required for testis development contains two genes related to known sexual regulators. Hum. Mol. Genet. 8, 989–996 ( 1999).
Nanda, I. et al. 300 million years of conserved synteny between chicken Z and human chromosome 9. Nature Genet. 21, 258 –259 (1999).
Raymond, C. S., Murphy, M. W., O'Sullivan, M. G., Bardwell, V. J. & Zarkower, D. Dmrt1, a gene related to worm and fly sexual regulators, is required for mammalian testis differentiation. Genes Dev. 14, 2587 –2595 (2000).A mouse knockout shows that Dmrt1 is required for postnatal testis differentiation, by affecting both Sertoli-cell and germ-cell development.
Crocker, M., Coghill, S. B. & Cortinho, R. An unbalanced autosomal translocation (7;9) associated with feminization. Clin. Genet. 34, 70– 73 (1988).
Ogata, T. et al. Impaired male sex development in an infant with molecularly defined partial 9p monosomy: implication for a testis forming gene(s) on 9p. J. Med. Genet. 34, 331–334 (1997).
Ion, R. et al. Failure of testicular development associated with a rearrangement of 9p24.1 proximal to the SNF2 gene. Hum. Genet. 102, 151–156 (1998).
Ottolenghi, C. et al. The region on 9p associated with 46,XY sex reversal contains several transcripts expressed in the urogenital system and a novel doublesex -related domain. Genomics 64, 170– 178 (2000).
Wilkins, A. S. Moving up the hierarchy: a hypothesis on the evolution of a genetic sex determination pathway. BioEssays 17, 71– 77 (1995).Proposes that sex-determining pathways, particularly those of nematodes, have evolved by the sequential addition of upstream regulators, so helping to explain the extreme molecular differences between these pathways and the evolution of the negatively acting Caenorhabditis elegans pathway.
Waxman, D. & Peck, J. R. Pleiotropy and the preservation of perfection. Science 279, 1210– 1213 (1998).
Kopp, A., Duncan, I. & Carroll, S. B. Genetic control and evolution of sexually dimorphic characters in Drosophila. Nature 408, 553–559 (2000).Shows how the differential regulation of the bric-a-brac (bab ) gene by Dsx and AbdB contributes to sexually dimorphic patterning in Drosophila species and how sexual selection is likely to have affected the evolution of bab regulation.
Hodgkin, J. A. & Brenner, S. Mutations causing transformation of sexual phenotype in the nematode Caenorhabditis elegans . Genetics 86, 275– 287 (1977).
Kenyon, C. A gene involved in the development of the posterior body region of C. elegans . Cell 46, 477–487 (1986).
Zhao, C. & Emmons, S. W. A transcription factor controlling development of peripheral sense organs in C. elegans. Nature 373, 74–78 ( 1995).
Emmons, S. W. in Cell Lineage and Fate Determination (ed. Moody, S. A.) 139– 155 (Academic, San Diego, California, 1999).
Wrischnik, L. A. & Kenyon, C. J. The role of lin-22, a hairy/enhancer of split homolog, in patterning the peripheral nervous system of C. elegans. Development 124, 2875–2888 ( 1997).
Branford, W. W., Benson, G. V., Ma, L., Maas, R. L. & Potter, S. S. Characterization of Hoxa-10/Hoxa-11 transheterozygotes reveals functional redundancy and regulatory interactions. Dev. Biol. 224, 373–387 ( 2000).
Hsieh-Li, H. M. et al. Hoxa11 structure, extensive antisense transcription, and function in male and female fertility. Development 121, 1373–1385 (1995).
Rijli, F. M. et al. Cryptorchidism and homeotic transformations of spinal nerves and vertebrae in Hoxa-10 mutant mice. Proc. Natl Acad. Sci. USA 92, 8185–8189 ( 1995).
Satokata, I., Benson, G. & Maas, R. Sexually dimorphic sterility phenotypes in Hoxa10-deficient mice. Nature 374, 460–463 (1995).
Katoh-Fukui, Y. et al. Male-to-female sex reversal in M33 mutant mice. Nature 393, 688–692 ( 1998).
Conradt, B. & Horvitz, H. R. The C. elegans protein EGL-1 is required for programmed cell death and interacts with the Bcl-2-like protein CED-9. Cell 93, 519– 529 (1998).
Sulston, J. E. & Horvitz, H. R. Post-embryonic cell lineages of the nematode Caenorhabditis elegans. Dev. Biol. 56, 110–156 ( 1977).
Trent, C., Tsung, N. & Horvitz, H. R. Egg-laying defective mutants of the nematode Caenorhabditis elegans. Genetics 104, 619–647 (1983).
Conradt, B. & Horvitz, H. R. The TRA-1A sex determination protein of C. elegans regulates sexually dimorphic cell deaths by repressing the egl-1 cell death activator gene. Cell 98 , 317–327 (1999). An impressive genetic demonstration in C. elegans that TRA-1 regulates the sexually dimorphic expression of egl-1 in one cell type by acting on an enhancer element located 5.6 kb downstream of the egl-1 coding region.
Ambros, V. & Horvitz, H. R. Heterochronic mutants of the nematode Caenorhabditis elegans. Science 226 , 409–416 (1984).
Arango, N. A., Lovell-Badge, R. & Behringer, R. R. Targeted mutagenesis of the endogenous mouse Mis promoter: in vivo definition of genetic pathways of vertebrate sexual development. Cell 99, 409– 419 (1999).An elegant study using in vivo mutagenesis of conserved binding sites in the Mis promoter and careful quantification of Mis expression to assess the importance of its regulation by Sf1 and Sox9. Shows that the Sox9 site is essential for Mis expression and that the Sf1 site is less important.
Viger, R. S., Mertineit, C., Trasler, J. M. & Nemer, M. Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonadal development and is a potent activator of the Müllerian inhibiting substance promoter. Development 125, 2665–2675 (1998).
Waterbury, J. A., Jackson, L. L. & Schedl, P. Analysis of the doublesex female protein in Drosophila melanogaster: role on sexual differentiation and behavior and dependence on intersex. Genetics 152, 1653–1667 (1999).
Jiang, M. et al. Genome-wide analysis of developmental and sex-regulated gene expression profiles in Caenorhabditis elegans. Proc. Natl Acad. Sci. USA 98, 218–223 (2001).
Bowles, J., Bullejos, M. & Koopman, P. A subtractive gene expression screen suggests a role for vanin-1 in testis development in mice. Genesis 27, 124–135 (2000).
Hodgkin, J. Two types of sex determination in a nematode. Nature 304, 267–268 (1983). A simple and effective demonstration that the sex-determination mechanism can be changed from XX/XO to ZZ/ZW by a pair of mutations in a single gene, resulting in a stable and fertile strain.
Miller, L. M., Plenefisch, J. D., Casson, L. P. & Meyer, B. J. xol-1: a gene that controls the male modes of both sex determination and X chromosome dosage compensation in C. elegans. Cell 55, 167–183 (1988).
Meyer, B. J. Sex in the worm; counting and compensating X-chromosome dose. Trends Genet. 16, 247–253 (2000).
Gray, S., Szymanski, P. & Levine, M. Short-range repression permits multiple enhancers to function autonomously within a complex promoter. Genes Dev. 8, 1829–1838 (1994).
Gray, S. & Levine, M. Transcriptional repression in development . Curr. Opin. Cell Biol. 8, 358– 364 (1996).
Small, S., Arnosti, D. N. & Levine, M. Spacing ensures autonomous expression of different stripe enhancers in the even-skipped promoter. Development 199, 762–772 (1993).
Chen, P. & Ellis, R. E. TRA-1A regulates transcription of fog-3, which controls germ cell fate in C. elegans. Development 127, 3119–3129 (2000).
Cai, H. N., Arnosti, D. N. & Levine, M. Long-range repression in the Drosophila embryo . Proc. Natl Acad. Sci. USA 93, 9309– 9314 (1996).
Usui-Aoki, K. et al. Formation of the male-specific muscle in female Drosophila by ectopic fruitless expression. Nature Cell Biol. 2, 500–506 ( 2000).
Janzen, F. J. & Paukstis, G. L. Environmental sex determination in reptiles: ecology, evolution, and experimental design . Q. Rev. Biol. 66, 149– 179 (1991).
Acknowledgements
The author thanks V. Bardwell, B. Charlesworth, E. Coucouvanis, J. Simon and three referees for critical reading of the manuscript; S. Carroll, A. Kopp and J. Hodgkin for sharing results before publication; and apologizes to those whose work could not be cited due to space limitations. Work in the Zarkower laboratory is supported by grants from the National Institutes of Health.
Author information
Authors and Affiliations
Related links
Related links
DATABASE LINKS
FURTHER INFORMATION
ENCYCLOPEDIA OF LIFE SCIENCES
Glossary
- BDELLOID ROTIFER
-
(or simply bdelloids, from the Greek word for leech). Microscopic aquatic animals. Most species creep on a surface by alternately moving their front and back ends, such as in an inchworm or a leech.
- HETEROGAMETIC SEX
-
The sex that produces gametes with different sex chromosomes. In XX/XY sex determination, the male is heterogametic, and in ZZ/ZW sex determination, the female is heterogametic.
- G-BANDING
-
(Giemsa banding). One of several methods for staining chromosomes, which produces light and dark bands characteristic for each homologous chromosome pair, so that individual chromosomes can be distinguished and examined for abnormalities in structure and number.
- RNA INTERFERENCE
-
(RNAi). This is a process by which double-stranded RNA specifically silences the expression of homologous genes through degradation of their cognate mRNA.
- SEX COMBS
-
Specialized rows of sensory bristles on the foreleg of male fruitflies, which are used during courtship to grip the female.
- SENSORY RAYS
-
Specialized sense organs in the nematode male tail, consisting of two sensory neurons and a structural cell. Used to locate and copulate with females.
- SERTOLI CELLS
-
Cells in the testis that produce secreted factors, including Müllerian inhibiting substance, and act as 'nurse cells' to provide nourishment to the early sperm cells.
- HOX PROTEINS
-
Transcription factors that are expressed in specific patterns, which are important for determining regional identity along the anterioposterior axis in the embryo.
- DEUTEROSTOME/PROTOSTOME
-
The two principal divisions of the animal phyla, based on how the mouth forms in the embryo.
- PLEIOTROPY
-
The phenomenon in which a single gene is responsible for several distinct and seemingly unrelated phenotypic effects.
- HYPODERMIS
-
The cellular layer that underlies and secretes the cuticle that forms the outer coating of a nematode or arthropod.
- BASIC HELIX–LOOP–HELIX
-
A common dimerization and DNA-binding motif. The helices mediate dimerization, and the adjacent basic region is required for DNA binding.
- CRYPTORCHIDISM
-
The failure of the testicles to descend from the abdominal cavity into the scrotum by one year of age (in humans).
- HETEROCHRONY
-
An evolutionary change in phenotype based on an alteration in the timing of developmental events.
Rights and permissions
About this article
Cite this article
Zarkower, D. Establishing sexual dimorphism: conservation amidst diversity?. Nat Rev Genet 2, 175–185 (2001). https://doi.org/10.1038/35056032
Issue Date:
DOI: https://doi.org/10.1038/35056032