Abstract
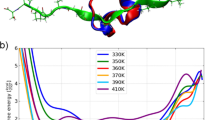
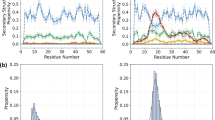
Determining how a protein folds is a central problem in structural biology. The rate of folding of many proteins is determined by the transition state, so that a knowledge of its structure is essential for understanding the protein folding reaction. Here we use mutation measurements—which determine the role of individual residues in stabilizing the transition state1,2—as restraints in a Monte Carlo sampling procedure to determine the ensemble of structures that make up the transition state. We apply this approach to the experimental data for the 98-residue protein acylphosphatase3, and obtain a transition-state ensemble with the native-state topology and an average root-mean-square deviation of 6 Å from the native structure. Although about 20 residues with small positional fluctuations form the structural core of this transition state, the native-like contact network of only three of these residues is sufficient to determine the overall fold of the protein. This result reveals how a nucleation mechanism involving a small number of key residues can lead to folding of a polypeptide chain to its unique native-state structure.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Matouschek, A., Kellis, J. T., Serrano, L. & Fersht, A. R. Mapping the transition state and pathway of protein folding by protein engineering. Nature 340, 122–126 (1989).
Fersht, A. R. Structure and Mechanism in Protein Science: A Guide to Enzyme Catalysis and Protein Folding (Freeman, New York, 1999).
Chiti, F. et al. Mutational analysis of acylphosphatase suggests the importance of topology and contact order in protein folding. Nature Struct. Biol. 6, 1005–1009 (1999).
Polanyi, J. C. & Zewail, A. H. Direct observation of the transition state. Acc. Chem. Res. 28, 119–132 (1995).
Winter, G., Fersht, A. R., Wilkinson, A. J., Zoller, M. & Smith, M. Redesigning enzyme structure by site-directed mutagenesis. Tyrosyl tRNA ATP binding. Nature 299, 756–758 (1982).
Fersht, A. R., Leatherbarrow, R. J. & Wells, T. N. Structure-activity relationships in engineered proteins. Analysis of use of binding energy by linear free energy relationships. Biochemistry 26, 6030–6038 (1987).
Wüthrich, K. Protein structure determination in solution by nuclear magnetic resonance spectroscopy. Science 243, 45–50 (1989).
Villegas, V., Martínez, J. C., Avilés, F. X. & Serrano, L. Structure of the transition state in the folding process of human procarboxypeptidase A2 activation domain. J. Mol. Biol. 283, 1027–1036 (1998).
Kuwajima, K. The molten globule state of α-lactalbumin. FASEB J. 10, 102–109 (1996).
Taddei, N. et al. Stabilisation of α-helices by site-directed mutagenesis reveals the secondary structure in the transition state for acylphosphatase folding. J. Mol. biol. 300, 633–647 (2000).
Plaxco, K. W., Simons, K. & Baker, D. Contact order, transition state placement and the refolding rate of single domain proteins. J. Mol. Biol. 277, 985–994 (1998).
Fersht, A. R., Itzhaki, L. S., elMasry, N. F., Matthews, J. M. & Otzen, D. E. Single versus parallel pathways of protein folding and fractional structure in the transition state. Proc. Natl Acad. Sci. USA 91, 10426–10429 (1994).
Daggett, V., Li, A., Itzhaki, L. S., Otzen, D. E. & Fersht, A. R. Structure of the transition state for folding of a protein derived from experiment and simulation. J. Mol. Biol. 257, 430–440 (1996).
Lazaridis, T. & Karplus, M. “New View” of protein folding reconciled with the old through multiple unfolding simulations. Science 278, 1928–1931 (1997).
Boczko, E. M. & Brooks, C. L. First-principles calculation of the folding free energy of a three-helix bundle protein. Science 269, 393–396 (1995).
Muñoz, V. & Eaton, W. A. A simple model for calculating the kinetics of protein folding from three-dimensional structures. Proc. Natl Acad. Sci. USA 96, 11311–11316 (1999).
Galzitskaya, O. V. & Finkelstein, A. V. A theoretical search for folding/unfolding nuclei in three-dimensional protein structures. Proc. Natl Acad. Sci. USA 96, 11299–11304 (1999).
Alm, E. & Baker, D. Prediction of protein-folding mechanisms from free-energy landscapes derived from native structures. Proc. Natl Acad. Sci. USA 96, 11305–11310 (1999).
Micheletti, C., Banavar, J. R., Maritan, A. & Seno, F. Protein structures and optimal folding from a geometrical variational principle. Phys. Rev. Lett. 82, 3372–3376 (1999).
Shoemaker, B. A., Wang, J. & Wolynes, P. G. Exploring structures in protein folding funnels with free energy functionals: The transition state ensemble. J. Mol. Biol. 287, 675–694 (1999).
Clementi, C., Jennings, P. A. & Onuchic, J. N. How native-state topology affects the folding of dihydrofolate reductase. Proc. Natl Acad. Sci. USA 97, 5871–5876 (2000).
Li, L., Mirny, L. A. & Shakhnovich, E. I. Kinetics, thermodynamics and evolution of non-native interactions in a protein folding nucleus. Nature Struct. Biol. 7, 336–342 (2000).
Li, A. & Daggett, V. Characterization of the transition state of protein unfolding by use of molecular dynamics: Chymotrypsin inhibitor 2. Proc. Natl Acad. Sci. USA 91, 10430–10434 (1994).
Vendruscolo, M. & Domany, E. Pairwise contact potentials are unsuitable for protein folding. J. Chem. Phys. 109, 11101–11108 (1998).
Vendruscolo, M. & Domany, E. Efficient dynamics in the space of contact maps. Fold. Des. 3, 329–336 (1998).
Zhou, Y. & Karplus, M. Interpreting the folding kinetics of helical proteins. Nature 401, 400–403 (1999).
Tanford, C. Protein denaturation. C. Theoretical models for the mechanism of denaturation. Adv. Protein Chem. 24, 1–95 (1970).
Berry, R. S., Beck, T. L., Davis, H. L. & Jellinek, J. in Advances in Chemical Physics Vol 70, (eds Prigogine, I. & Rice, S. A.) 75–135 (Wiley, New York, 1988).
Zhou, Y., Vitkup, D. & Karplus, M. Native proteins are surface-molten solids: Application of the Lindemann criterion for the solid versus liquid state. J. Mol. Biol. 285, 1371–1375 (1999).
Domany, E. Superparamagnetic clustering of data. The definitive solution of an ill posed problem. Physica A 263, 158–169 (1999).
Acknowledgements
We thank F. Chiti and A. Fersht for discussions and comments on this work. We also thank G. Ramponi and N. Taddei for continuing collaborations involving AcP. The Oxford Centre for Molecular Science is supported by BBSRC, EPSRC and MRC. M.V. is supported by an EMBP long-term fellowship. E.P. is supported in Oxford by an EC fellowship. C.M.D. is supported in part by a programme grant from the Wellcome Trust. M.K. Thanks Oxford University for inviting him to spend a year as Eastman Visiting Professor. Much of this work was done while he was in Oxford; the part done at Harvard was supported in part by the National Institutes of Health.
Author information
Authors and Affiliations
Corresponding authors
Supplementary information
Rights and permissions
About this article
Cite this article
Vendruscolo, M., Paci, E., Dobson, C. et al. Three key residues form a critical contact network in a protein folding transition state. Nature 409, 641–645 (2001). https://doi.org/10.1038/35054591
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/35054591
This article is cited by
-
Exploring the conformational dynamics and flexibility of intrinsically disordered HIV-1 Nef protein using molecular dynamic network approaches
3 Biotech (2021)
-
Validation and quality assessment of macromolecular structures using complex network analysis
Scientific Reports (2019)
-
Discrimination power of knowledge-based potential dictated by the dominant energies in native protein structures
Amino Acids (2019)
-
Normal mode analysis as a method to derive protein dynamics information from the Protein Data Bank
Biophysical Reviews (2017)
-
Molecular Recognition by Templated Folding of an Intrinsically Disordered Protein
Scientific Reports (2016)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.