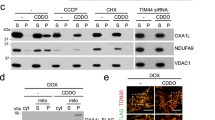
Abstract
HEATSHOCK protein 60 (hsp60) in the matrix of mitochondria is essential for the folding and assembly of newly imported proteins1. Hsp60 belongs to a class of structurally related chaperonins found in organelles of endosymbiotic origin and in the bacterial cytosol2–9. Hsp60 monomers form a complex arranged as two stacked 7-mer rings6. This 14-mer complex binds unfolded proteins at its surface, then seems to catalyse their folding in an ATP-dependent process10. The question arises as to how such an assembly machinery is itself folded and assembled. Hsp60 subunits are encoded by a nuclear gene and translated in the cytosol as precursors7 which are translocated into mitochondria and proteolytically processed. In both intact cells and isolated mitochondria of the hsp60-defective yeast mutant mif4, self-assembly of newly imported wild-type subunits is not observed. Functional pre-existing hsp60 complex is required in order to form new, assembled, 14-mer. Subunits imported in vitro are assembled with a surprisingly fast half-time of 5–10 min, indicative of a catalysed reaction. These findings are further evidence that self-assembly may not be the principal mechanism by which proteins attain their functional conformation in the intact cell.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Cheng, M. Y. et al. Nature 337, 620–625 (1989).
Laskey, R. A., Honda, B. M., Mills, A. D. & Finch, J. T. Nature 275, 416–420 (1978).
Ellis, R. J. & Hemmingsen, S. M. Trends biochem. Sci. 14, 339–342 (1989).
Rothman, J. E. Cell 59, 591–601 (1989).
Barraclough, R. & Ellis, R. J. Biochim. biophys. Acta 608, 19–31 (1980).
McMullen, T. W. & Hallberg, R. L. Molec. cell. Biol. 8, 371–380 (1988).
Reading, D. S., Hallberg, R. L. & Myers, A. M. Nature 337, 655–659 (1989).
Hemmingsen, S. M. et al. Nature 333, 330–334 (1988).
Goloubinoff, P., Christeller, J. T., Gatenby, A. A. & Lorimer, G. H. Nature 342, 884–889 (1989).
Ostermann, J., Horwich, A. L., Neupert, W. & Hartl, F.-U. Nature 341, 125–130 (1989).
Luck, D. J. L. J. Cell Biol. 24, 461–470 (1965).
Hartl, F.-U. & Neupert, W. Science 247, 930–938 (1990).
Laemmli, U. K. Nature 227, 680–685 (1970).
Cheng, M. Y. thesis, Yale Univ. (1990).
Pelham, H. R. B. & Jackson, R. J. Eur. J. Biochem. 67, 247–256 (1976).
Towbin, H., Staehelin, T. & Gordon, J. Proc. natn. Acad. Sci. U.S.A. 76, 4350–4354 (1979).
Musgrove, J. E., Johnson, R. A. & Ellis, R. J. Eur. J. Biochem. 163, 529–543 (1987).
Bahr, G. F. & Zeitler, E. J. Cell Biol. 15, 489–501 (1962).
Lissin, N. M., Venyaminoy, S. Y. & Girshovich, A. S. Nature 348, 339–342 (1990).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Cheng, M., Hartl, FU. & Norwich, A. The mitochondrial chaperonin hsp60 is required for its own assembly. Nature 348, 455–458 (1990). https://doi.org/10.1038/348455a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/348455a0
This article is cited by
-
Post-COVID-19 Hyposmia Does Not Exhibit Main Neurodegeneration Markers in the Olfactory Pathway
Molecular Neurobiology (2024)
-
Molecular Chaperones as Therapeutic Target: Hallmark of Neurodegenerative Disorders
Molecular Neurobiology (2023)
-
Small molecule inhibitors of the mitochondrial ClpXP protease possess cytostatic potential and re-sensitize chemo-resistant cancers
Scientific Reports (2021)
-
Anti-mitochondrial autoantibodies in systemic lupus erythematosus and their association with disease manifestations
Scientific Reports (2019)
-
HSP60 expression profile under different extreme temperature stress in albino northern snakehead, Channa argus
Cell Stress and Chaperones (2018)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.