Abstract
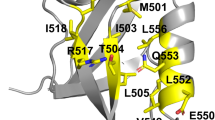
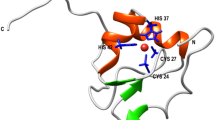
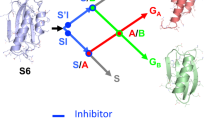
In the transition state for unfolding of barnase, the hydrophobic core between the major α-helix and β-sheet is somewhat weakened, the C terminus of the major helix is largely intact but its N terminus is exposed and a major loop has been invaded by solvent.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Creighton, T. E. Proc. natn. Acad. Sci. U.S.A. 85, 5082–5086 (1988).
Udgaonkar, J. B. & Baldwin, R. L. Nature 335, 694–699 (1988).
Roder, H., Elöve, G. A. & Englander, S. W. Nature 335, 700–704 (1988).
Beasty, A. M. et al. Biochemistry 25, 2965–2974 (1989).
Garvey, E. P. & Matthews, C. R. Biochemistry 28, 2083–2093 (1989).
Chen, B.-l., Baase, W. A. & Schellman, J. A. Biochemistry 28, 691–699 (1989).
Goldenberg, D. P., Frieden, R. W., Haack, J. A. & Morrison, T. B. Nature 338, 127–132 (1989).
Winter, G., Fersht, A. R., Wilkinson, A. J., Zoller, M. & Smith, M. Nature 299, 756–758 (1982).
Fersht, A. R. Biochemistry 26, 8031–8037 (1987).
Kellis, J. T. Jr, Nyberg, K., Sali, D. & Fersht, A. R. Nature 333, 784–786 (1988).
Sali, D., Bycroft, M. & Fersht, A. R. Nature 335, 741–743 (1988).
Kellis, J. T. Jr, Nyberg, K. & Fersht, A. R. Biochemistry 28, 4914–4922 (1989).
Mauguen, Y. et al. Nature 297, 162–164 (1982).
Schmid, F. X. & Baldwin, R. L. Proc. natn. Acad. Sci. U.S.A. 75, 4764–4768 (1978).
Fersht, A. R., Leatherbarrow, R. J. & Wells, T. N. C. Biochemistry 26, 6030–6038 (1987).
Tanford, C. Adv. Protein Chem. 24, 1–95 (1970).
Fersht, A. R. Enzyme Structure and Mechanism 2nd edn (Freeman, New York, 1985).
Fersht, A. R. Biochemistry 27, 1577–1580 (1988).
Wolfenden, R., Andersson, L., Cullis, P. M. & Southgate, C. C. B. Biochemistry 20, 849–855 (1981).
Presta, L. G. & Rose, G. D. Science 240, 1632–1641 (1988).
Richardson, J. S. & Richardson, D. C. Science 240, 1648–1652 (1988).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Matouschek, A., Kellis, J., Serrano, L. et al. Mapping the transition state and pathway of protein folding by protein engineering. Nature 340, 122–126 (1989). https://doi.org/10.1038/340122a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/340122a0
This article is cited by
-
Protein folding rate evolution upon mutations
Biophysical Reviews (2023)
-
Thermodynamics of protein folding: methodology, data analysis and interpretation of data
European Biophysics Journal (2019)
-
Large enhancement of response times of a protein conformational switch by computational design
Nature Communications (2018)
-
Effects of pH and aggregation in the human prion conversion into scrapie form: a study using molecular dynamics with excited normal modes
European Biophysics Journal (2018)
-
Outer membrane protein folding from an energy landscape perspective
BMC Biology (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.