Abstract
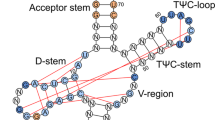
The genetic code is determined by both the specificity of the triplet anticodon of tRNAs for codons in mRNAs and the specificity with which tRNAs are charged with amino acids. The latter depends on interactions between tRNAs and their charging enzymes, and an advance in understanding such interactions was provided recently by the demonstration that a major determinant of the identity of alanine tRNA is located in the amino-acid acceptor helix. Multiple substitutions in many distinct parts of the molecule do not prevent aminoacylation with alanine1. Substitution of the G3·U70 base pair with G3·C70 or A3·U70 in the acceptor helix prevents aminoacylation in vivo and in vitro, however1, and the introduction of this base pair into tRNACys (ref. 1) or tRNAPhe (refs 1, 2) enables both to accept alanine. The importance of a single base pair in the acceptor helix and the results of recent footprinting experiments3 prompted us to investigate the possibility that a minihelix, composed only of the amino-acid acceptor-TΨC helix, could be a substrate for alanine tRNA synthetase. We show here that a synthetic hairpin minihelix can be enzymatically aminoacylated with alanine. Alanine incorporation requires a single G·U base pair, and occurs in helices that otherwise differ significantly in sequence. Aminoacylation can be achieved with only seven base pairs in the helix.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Hou, Y.-M. & Schimmel, P. Nature 333, 140–145 (1988).
McClain, W. H. & Foss, K. Science 240, 793–796 (1988).
Park, S. J. & Schimmel, P. J. biol. Chem. 263, 16527–16530 (1988).
Sprinzl, M., Hartmann, T., Meissner, F., Moll, H. & Vorderwullbecke, T. Nucleic Acids Res. 15, r53–rl88 (1988).
Park, S. J., Hou, Y.-M. & Schimmel, P. Biochemistry (in the press).
Schulman, L. H. & Pelka, H. Biochemistry 24, 7309–7314 (1985).
Rogers, M. J. & Soll, D. Proc. natn. Acad. Sci. U.S.A. 85, 6627–6631 (1988).
Schulman, L. H. & Pelka, H. Science 242, 765–768 (1988).
Muramatsu, T. et al. Nature 336, 179–181 (1988).
Sampson, J. K., DeRenzo, A., Behlen, L. & Uhlenbeck, O. C. Science (in the press).
Imura, N., Weiss, G. B. & Chambers, R. W. Nature 222, 1147–1148 (1969).
Schimmel, P. R. & Soll, D. A. Rev. Biochem. 48, 601–648 (1979).
Anderson, S. et al. Nature 290, 457–465 (1981).
de Bruijn, M. H. L. & Klug, A. EMBO J. 2, 1309–1321 (1983).
McClain, W. H., Guerrier-Takada, C. & Altman, S. Science 238, 527–530 (1987).
de Duve, C. Nature 333, 117–118 (1988).
Yarus, M. Science 240, 1751–1758 (1988).
Weiner, A. M. & Maizels, N. Proc. natn. Acad. Sci. U.S.A. 84, 7383–7387 (1987).
Fersht, A. R. et al. Biochemistry 14, 1–4 (1975).
Hill, K. & Schimmel, P. Biochemistry (in the press).
Jasin M., Regan, L. & Schimmel, P. J. biol. Chem 260, 2226–2230 (1985).
Milligan, J. F., Groebe, D. R., Witherell, G. W. & Uhlenbeck, O. C. Nucleic Acids Res. 15, 8783–8798 (1987).
Samson, J. & Uhlenbeck, O. C. Proc. natn. Acad. Sci. U.S.A. 85, 1033–1037 (1988).
Grodberg, J. D. & Dunn, J. J. J. Bact. 170, 1245–1253 (1988).
England, T. E. & Uhlenbeck, O. C. Nature 275, 560–561 (1978).
Silberklang, M., Gillum, A. M. & RajBhandary, U. L. Meth Enzym. 59, 58–109 (1979).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Francklyn, C., Schimmel, P. Aminoacylation of RNA minihelices with alanine. Nature 337, 478–481 (1989). https://doi.org/10.1038/337478a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/337478a0
This article is cited by
-
G:U-Independent RNA Minihelix Aminoacylation by Nanoarchaeum equitans Alanyl-tRNA Synthetase: An Insight into the Evolution of Aminoacyl-tRNA Synthetases
Journal of Molecular Evolution (2020)
-
The Emergence of Life
Space Science Reviews (2019)
-
The emerging complexity of the tRNA world: mammalian tRNAs beyond protein synthesis
Nature Reviews Molecular Cell Biology (2018)
-
Exploration of RNA Sequence Space in the Absence of a Replicase
Journal of Molecular Evolution (2018)
-
The Rodin-Ohno hypothesis that two enzyme superfamilies descended from one ancestral gene: an unlikely scenario for the origins of translation that will not be dismissed
Biology Direct (2014)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.