Abstract
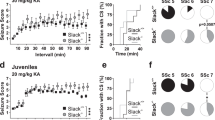
l-glutamate, the neurotransmitter of the majority of excitatory synapses in the brain, acts on three classes of ionotropic receptors: NMDA (N-methyl-d-aspartate), AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid) and kainate receptors. Little is known about the physiological role of kainate receptors because in many experimental situations it is not possible to distinguish them from AMPA receptors1,2. Mice with disrupted kainate receptor genes enable the study of the specific role of kainate receptors in synaptic transmission as well as in the neurotoxic effects of kainate. We have now generated mutant mice lacking the kainate-receptor subunit GluR6. The hippocampal neurons in the CA3 region of these mutant mice are much less sensitive to kainate. In addition, a postsynaptic kainate current evoked in CA3 neurons by a train of stimulation of the mossy fibre system is absent in the mutant3,4. We find that GluR6-deficient mice are less susceptible to systemic administration of kainate, as judged by onset of seizures and by the activation of immediate early genes in the hippocampus. Our results indicate that kainate receptors containing the GluR6 subunit are important in synaptic transmission as well as in the epileptogenic effects of kainate.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Bettler, B. & Mulle, C. AMPA and kainate receptors. Neuropharmacology 34, 123–139 (1995).
Lerma, J., Morales, M., Vicente, M. A. & Herreras, O. Glutamate receptors of the kainate type and synaptic transmission. Trends Neurosci. 20, 9–12 (1997).
Castillo, P. E., Malenka, R. C. & Nicoll, R. A. Kainate receptors mediate a slow postsynaptic current in hippocampal CA3 neurons. Nature 388, 182–186 (1997).
Vignes, M. & Collingridge, G. L. The synaptic activation of kainate receptors. Nature 388, 179–182 (1997).
Egebjerg, J., Bettler, B., Hermans-Borgmeyer, I. & Heinemann, S. Cloning of a cDNA for a glutamate receptor subunit activated by kainate but not AMPA. Nature 351, 745–748 (1991).
Raymond, L. A., Blackstone, C. D. & Huganir, R. L. Phosphorylation and modulation of recombinant GluR6 glutamate receptors by cAMP-dependent protein kinase. Nature 361, 637–641 (1993).
Werner, P., Voigt, M., Keinänen, K., Wisden, W. & Seeburg, P. H. Cloning of a putative high-affinity kainate receptor expressed predominantly in hippocampal CA3 cells. Nature 351, 742–744 (1991).
Robinson, J. H. & Deadwyler, S. A. Kainic acid produces depolarization of CA3 pyramidal cells in the in vitro hippocampal slices. Brain Res. 221, 117–127 (1981).
Zucker, R. Short-term synaptic plasticity. Annu. Rev. Neurosci. 12, 13–31 (1989).
Salin, P., Scanziani, M., Malenka, R. & Nicoll, R. Distinct short-term plasticity at two excitatory synapses in the hippocampus. Proc. Natl Acad. Sci. USA 93, 13304–13309 (1996).
Kamiya, H., Shinozaki, H. & Yamamoto, C. Activation of metabotropic glutamate receptor type 2/3 suppresses transmission at rat hippocampal mossy fibre synapse. J. Physiol. 493, 447–455 (1996).
Ben-Ari, Y. Limbic seizure and brain damage produced by kainic acid: mechanisms and relevance to human temporal lobe epilepsy. Neuroscience 14, 375–403 (1985).
Nadler, J. Intraventricular kainic acid preferentially destroys hippocampal pyramidal cells. Nature 271, 676–677 (1978).
Schauwecker, P. & Steward, O. Genetic determinants of susceptibility to excitotoxic cell death: implications for gene targeting approaches. Proc. Natl Acad. Sci. USA 94, 4103–4108 (1997).
Le Gal La Salle, G. Long-lasting and sequential increase in c-fos oncoprotein expression in kainic-acid induced status epilepticus. Neurosci. Lett. 88, 127–130 (1988).
Smeyne, R. J.et al. Continuous c-fos expression precedes programmed cell death in vivo. Nature 363, 166–169 (1993) (erratum, ibid. 365, 279; (1993).
Chittajallu, R.et al. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature 379, 78–81 (1996).
Rodriguez-Moreno, A., Herrera, O. & Lerma, J. Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron 19, 893–901 (1997).
Clarke, V. R. J.et al. Ahippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature 389, 599–603 (1997).
Vetter, D. E.et al. Inner ear defects induced by null mutation of the isk gene. Neuron 17, 1251–1264 (1996).
Bischoff, S., Barhanin, J., Bettler, B., Mulle, C. & Heinemann, S. Spatial distribution of kainate receptor subunit mRNA in the mouse basal ganglia and ventral mesencephalon. J. Comp. Neurol. 379, 541–562 (1997).
Forrest, D.et al. Targeted disruption of NMDA receptor 1 gene abolishes NMDA response and results in neonatal death. Neuron 13, 325–338 (1994).
Monaghan, D. & Cotman, C. The distribution of [3H]-kainic acid binding sites in rat CNS as determined by autoradiography. Brain Res. 252, 91–100 (1982).
Young, S., Porrino, L. & Iadarola, M. Cocaine induces striatal c-Fos-immunoreactive proteins via dopaminergic D1 receptors. Proc. Natl Acad. Sci. USA 88, 1291–1295 (1991).
Kempermann, G., Kuhn, H. G. & Gage, F. H. More hippocampal neurons in adult mice living in an enriched environment. Nature 386, 493–495 (1997).
Acknowledgements
We thank L. O'Leary, Q.-C. Phan, H. Garjeda, E.Normand and the animal research departments for technical assistance; B. Vissel for help in isolating the genomic clones; M. Iadarola for anti-Fra antibody; M. Allard for help with kainate autoradiography; and Eli Lilly for a gift of GYKI 53655. This work was supported by grants and fellowships from the CNRS, the French MRES, the NIH, the Fondation pour la Recherche Médicale, and the Région Aquitaine (to C.M.), the Schweizerische Nationalfond and the Deutsche Forschungsgemeinschaft (to A.S.), the Spanish MEC (to I.P.-O.), the Conycit-BID (to P.E.C.), a joint program project grant (to F.H.G. and S.F.H.), the NIH (NINDS) and theMcKnight foundation (to S.F.H.).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mulle, C., Sailer, A., Pérez-Otaño, I. et al. Altered synaptic physiology and reduced susceptibility to kainate-induced seizures in GluR6-deficient mice. Nature 392, 601–605 (1998). https://doi.org/10.1038/33408
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/33408
This article is cited by
-
The kainate receptor GluK2 mediates cold sensing in mice
Nature Neuroscience (2024)
-
Interneuronal GluK1 kainate receptors control maturation of GABAergic transmission and network synchrony in the hippocampus
Molecular Brain (2023)
-
Pharmacological inhibition of the inflammatory receptor CCR2 relieves the early deleterious consequences of status epilepticus
Scientific Reports (2023)
-
Genome-wide association study of the human brain functional connectome reveals strong vascular component underlying global network efficiency
Scientific Reports (2022)
-
Peripheral viral challenge increases c-fos level in cerebral neurons
Metabolic Brain Disease (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.