Abstract
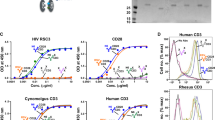
CD4 (T4) is a glycoprotein of relative molecular mass 55,000 (Mr 55K) on the surface of T lymphocytes which is thought to interact with class II MHC (major histocompatibility complex) molecules, mediating efficient association of helper T cells with antigenbearing targets1–3. The CD4 protein is also the receptor for HIV, a T-lymphotropic RNA virus responsible for the human acquired immune deficiency syndrome (AIDS) (refs 4–7). To define the mechanisms of interaction of CD4 with the surface of antigen-presenting cells and with HIV, we have isolated the CD4 gene and expressed this gene in several different cellular environments7,8. Here we describe an efficient expression system in which a recombinant, soluble form of CD4 (sCD4) is secreted into tissue culture supernatants. This scD4 retains the structural and biological properties of CD4 on the cell surface, binds to the envelope glycoprotein (gpllO) of HIV and inhibits the binding of virus to CD4+ lymphocytes, resulting in a striking inhibition of virus infectivity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 51 print issues and online access
$199.00 per year
only $3.90 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
Gay, D. et al. Nature 328, 626–629 (1987).
Sleckman, B. D. et al. Nature 328, 351–353 (1987).
Thomas, Y., Rognozinski, L. & Chess, L. Immun. Rev. 74, 113–128 (1985).
Dalgleish, A. G. et al. Nature 312, 763–766 (1984).
Klatzman, D. et al. Nature 312, 767–768 (1984).
McDougal, J. S. et al. Science 231, 382–385 (1986).
Maddon, P. J. et al. Cell 47, 333–348 (1986).
Maddon, P. J. et al. Cell 42, 93–104 (1985).
Maddon, P. J. et al. Proc. natn. Acad. Sci. U.S.A. (in the press).
Pfarr, D. S., Sathe, G. & Refi, M. F. DNA 4, 461–467 (1985).
Urlaud, G., Kas, E., Carothers, A. M. & Chasin, L. A. Cell 33, 405–412 (1983).
Kaufman, R. J., Sharp, P. A. & Latt, S. A. Molec. cell. Biol. 3, 699–711 (1983).
McDougal, J.-S. et al. J. immun. Meth. 76, 171–183 (1985).
Berg, P. E. et al. Molec. cell. Biol. 3, 1246–1254 (1983).
Subramani, S., Mulligan, R. & Berg, P. Molec. cell. Biol. 1, 854–864 (1981).
Wigler, M. et al. Cell 11, 223–232 (1977).
McDougal, J. S. et al. J. Immun. 135, 3151–3162 (1985).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Deen, K., McDougal, J., Inacker, R. et al. soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature 331, 82–84 (1988). https://doi.org/10.1038/331082a0
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1038/331082a0
This article is cited by
-
Profound structural conservation of chemically cross-linked HIV-1 envelope glycoprotein experimental vaccine antigens
npj Vaccines (2023)
-
Distinct conformations of the HIV-1 V3 loop crown are targetable for broad neutralization
Nature Communications (2021)
-
Deciphering the mystery of hepatitis B virus receptors: A historical perspective
VirusDisease (2015)
-
A streamlined implementation of the glutamine synthetase-based protein expression system
BMC Biotechnology (2013)
-
Targeting host cofactors to inhibit viral infection
Frontiers in Biology (2012)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.