Abstract
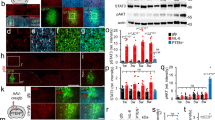
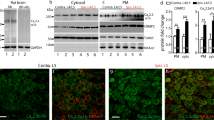
To dissect the molecular basis of the neuroimmune response associated with the genesis of inflammatory (nociceptive) pain, we constructed a herpes simplex virus-based gene transfer vector to express the antiinflammatory cytokine interleukin-10 (IL-10), and used it to examine the effect of IL-10 expression in activated microglial cells in vitro, and in inflammatory pain in vivo. IL-10 reduced the phosphorylation of p38 mitogen-activated protein kinase (MAPK) and decreased the expression of full-length membrane spanning tumor necrosis factor-α (mTNFα) following lipopolysaccharide stimulation of microglia in vitro. IL-10 also reduced intracellular cleavage of mTNFα and release of the soluble cleavage product sTNFα. Similar effects on TNFα expression were observed when the cells were pretreated with a p38 MAPK inhibitor. In animals, injection of a dilute solution of formalin in the skin resulted in an increase in mTNFα in spinal dorsal horn, without detectable sTNFα. Local release of IL-10 achieved by gene transfer reduced the number of spontaneous flinches in the early and delayed phases of the formalin test of inflammatory pain. The effect of IL-10 on nocisponsive behavior correlated with a block in phosphorylation of p38 and reduced expression of 26 kDa mTNFα in spinal microglia. The results emphasize the key role played by membrane TNFα in the spinal neuroimmune response in pain caused by peripheral inflammation.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Woolf CJ, Allchorne A, Safieh-Garabedian B, Poole S . Cytokines, nerve growth factor and inflammatory hyperalgesia: the contribution of tumour necrosis factor alpha. Br J Pharmacol 1997; 121: 417–424.
Sorkin LS, Xiao WH, Wagner R, Myers RR . Tumour necrosis factor-alpha induces ectopic activity in nociceptive primary afferent fibres. Neuroscience 1997; 81: 255–262.
Anzai H, Hamba M, Onda A, Konno S, Kikuchi S . Epidural application of nucleus pulposus enhances nociresponses of rat dorsal horn neurons. Spine 2002; 27: E50–E55.
Onda A, Hamba M, Yabuki S, Kikuchi S . Exogenous tumor necrosis factor-alpha induces abnormal discharges in rat dorsal horn neurons. Spine 2002; 27: 1618–1624; discussion 1624.
Sorkin LS, Doom CM . Epineurial application of TNF elicits an acute mechanical hyperalgesia in the awake rat. J Peripher Nerv Syst 2000; 5: 96–100.
Junger H, Sorkin LS . Nociceptive and inflammatory effects of subcutaneous TNFalpha. Pain 2000; 85: 145–151.
Watkins LR, Maier SF . Beyond neurons: evidence that immune and glial cells contribute to pathological pain states. Physiol Rev 2002; 82: 981–1011.
DeLeo JA, Tanga FY, Tawfik VL . Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist 2004; 10: 40–52.
Wieseler-Frank J, Maier SF, Watkins LR . Glial activation and pathological pain. Neurochem Int 2004; 45: 389–395.
Watkins LR, Milligan ED, Maier SF . Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv Exp Med Biol 2003; 521: 1–21.
Peng X, Zhou Z, Glorioso JC, Fink DJ, Mata M . Tumor necrosis factor alpha contributes to below-level neuropathic pain after spinal cord injury. Ann Neurol 2006; 59: 843–851.
Hao S, Mata M, Glorioso JC, Fink DJ . HSV-mediated expression of interleukin-4 in dorsal root ganglion neurons reduces neuropathic pain. Mol Pain 2006; 2: 6.
Samad TA, Sapirstein A, Woolf CJ . Prostanoids and pain: unraveling mechanisms and revealing therapeutic targets. Trends Mol Med 2002; 8: 390–396.
Reeve AJ, Patel S, Fox A, Walker K, Urban L . Intrathecally administered endotoxin or cytokines produce allodynia, hyperalgesia and changes in spinal cord neuronal responses to nociceptive stimuli in the rat. Eur J Pain 2000; 4: 247–257.
Milligan ED, Sloane EM, Langer SJ, Cruz PE, Chacur M, Spataro L et al. Controlling neuropathic pain by adeno-associated virus driven production of the anti-inflammatory cytokine, interleukin-10. Mol Pain 2005; 1: 9.
Milligan ED, Langer SJ, Sloane EM, He L, Wieseler-Frank J, O'Connor K et al. Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci 2005; 21: 2136–2148.
Liu J, Wolfe D, Hao S, Huang S, Glorioso JC, Mata M et al. Peripherally delivered glutamic acid decarboxylase gene therapy for spinal cord injury pain. Mol Ther 2004; 10: 57–66.
Hao S, Mata M, Wolfe D, Glorioso JC, Fink DJ . Gene transfer of glutamic acid decarboxylase reduces neuropathic pain. Ann Neurol 2005; 57: 914–918.
Cheepsunthorn P, Radov L, Menzies S, Reid J, Connor JR . Characterization of a novel brain-derived microglial cell line isolated from neonatal rat brain. Glia 2001; 35: 53–62.
Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH . The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol 1992; 107: 660–664.
Wagner R, Myers RR . Endoneurial injection of TNF-alpha produces neuropathic pain behaviors. Neuroreport 1996; 7: 2897–2901.
Schafers M, Sorkin LS, Sommer C . Intramuscular injection of tumor necrosis factor-alpha induces muscle hyperalgesia in rats. Pain 2003; 104: 579–588.
Schafers M, Sorkin LS, Geis C, Shubayev VI . Spinal nerve ligation induces transient upregulation of tumor necrosis factor receptors 1 and 2 in injured and adjacent uninjured dorsal root ganglia in the rat. Neurosci Lett 2003; 347: 179–182.
Schafers M, Svensson CI, Sommer C, Sorkin LS . Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci 2003; 23: 2517–2521.
Zelenka M, Schafers M, Sommer C . Intraneural injection of interleukin-1beta and tumor necrosis factor-alpha into rat sciatic nerve at physiological doses induces signs of neuropathic pain. Pain 2005; 116: 257–263.
Tjolsen A, Berge OG, Hunskaar S, Rosland JH, Hole K . The formalin test: an evaluation of the method. Pain 1992; 51: 5–17.
Watkins LR, Martin D, Ulrich P, Tracey KJ, Maier SF . Evidence for the involvement of spinal cord glia in subcutaneous formalin induced hyperalgesia in the rat. Pain 1997; 71: 225–235.
Solomon KA, Covington MB, DeCicco CP, Newton RC . The fate of pro-TNF-alpha following inhibition of metalloprotease-dependent processing to soluble TNF-alpha in human monocytes. J Immunol 1997; 159: 4524–4531.
Mohler KM, Sleath PR, Fitzner JN, Cerretti DP, Alderson M, Kerwar SS et al. Protection against a lethal dose of endotoxin by an inhibitor of tumour necrosis factor processing. Nature 1994; 370: 218–220.
Armstrong L, Thickett DR, Christie SJ, Kendall H, Millar AB . Increased expression of functionally active membrane-associated tumor necrosis factor in acute respiratory distress syndrome. Am J Respir Cell Mol Biol 2000; 22: 68–74.
Georgopoulos S, Plows D, Kollias G . Transmembrane TNF is sufficient to induce localized tissue toxicity and chronic inflammatory arthritis in transgenic mice. J Inflamm 1996; 46: 86–97.
Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD et al. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem 2003; 86: 1534–1544.
Kontoyiannis D, Kotlyarov A, Carballo E, Alexopoulou L, Blackshear PJ, Gaestel M et al. Interleukin-10 targets p38 MAPK to modulate ARE-dependent TNF mRNA translation and limit intestinal pathology. EMBO J 2001; 20: 3760–3770.
Pestka S, Krause CD, Sarkar D, Walter MR, Shi Y, Fisher PB . Interleukin-10 and related cytokines and receptors. Annu Rev Immunol 2004; 22: 929–979.
Vale ML, Marques JB, Moreira CA, Rocha FA, Ferreira SH, Poole S et al. Antinociceptive effects of interleukin-4, -10, and -13 on the writhing response in mice and zymosan-induced knee joint incapacitation in rats. J Pharmacol Exp Ther 2003; 304: 102–108.
Plunkett JA, Yu CG, Easton JM, Bethea JR, Yezierski RP . Effects of interleukin-10 (IL-10) on pain behavior and gene expression following excitotoxic spinal cord injury in the rat. Exp Neurol 2001; 168: 144–154.
Acknowledgements
This work was supported by grants from the National Institutes of Health (DJF and MM) and the Department of Veterans Affairs (DJF and MM). We thank Dr Joseph Glorioso (University of Pittsburgh) for providing the HSV vector backbone and 7b cells, Dr JR Connor (Pennsylvania State University College of Medicine) for the HAPI cells, Molly Rockstad for technical assistance, Vikram Thakur Singh for propagation of the vectors and Shue Liu for tissue culture.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, Z., Peng, X., Hao, S. et al. HSV-mediated transfer of interleukin-10 reduces inflammatory pain through modulation of membrane tumor necrosis factor α in spinal cord microglia. Gene Ther 15, 183–190 (2008). https://doi.org/10.1038/sj.gt.3303054
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3303054
Keywords
This article is cited by
-
Cytochrome P450 26A1 Contributes to the Maintenance of Neuropathic Pain
Neuroscience Bulletin (2023)
-
Current Gene Therapy using Viral Vectors for Chronic Pain
Molecular Pain (2015)
-
High cerebrospinal fluid levels of interleukin-10 attained by AAV in dogs
Gene Therapy (2015)