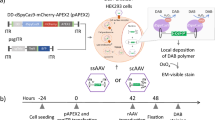
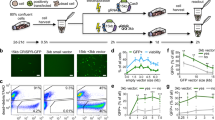
Abstract
Transcript depletion using small interfering RNA (siRNA) technology represents a potentially valuable technique for the treatment of cancer. However, delivering therapeutic quantities of siRNA into solid tumors by chemical transfection is not feasible, whereas viral vectors efficiently transduce many human tumor cell lines. Yet producing sufficient quantities of viral vectors that elicit acute and selective cytotoxicity remains a major obstacle for preclinical and clinical trials. Using the invertebrate Spodoptera frugiperda (Sf9) cell line, we were able to produce high titer stocks of cytotoxic recombinant adeno-associated virus (rAAV) that express short hairpin RNA (shRNA) and that efficiently deplete Hec1 (highly expressed in cancer 1), or Kntc2 (kinetochore-associated protein 2), a kinetochore protein directly involved in kinetochore microtubule interactions, chromosome congression and spindle checkpoint signaling. Depletion of Hec1 protein results in persistent spindle checkpoint activation followed by cell death. Because Hec1 expression and activity are only present in mitotic cells, non-dividing cells were not affected by rAAV treatment. On the basis of the results of screening 56 human tumor cell lines with three different serotype vectors, we used a tumor xenograft model to test the effects in vivo. The effects of the shHec1 vector were evident in sectioned and stained tumors. The experiments with rAAV-shRNA vectors demonstrate the utility of producing vectors in invertebrate cells to obtain sufficient concentrations and quantities for solid tumor therapy. This addresses an important requirement for cancer gene therapy, to produce cytotoxic vectors in sufficient quantities and concentrations to enable quantitative transduction and selective killing of solid tumor cells.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout











Similar content being viewed by others
Abbreviations
- BEV:
-
baculovirus expression vector
- CGNS:
-
cerebella granule neurons
- CNS:
-
central nervous system
- dsRNA:
-
double-strand RNA
- eGFP:
-
enhanced green fluorescence protein
- Hec1:
-
highly expressed in cancer 1
- hrGFP:
-
humanized Renilla green fluorescence protein
- HSPG:
-
heparan sulfate proteogylcans
- kmts:
-
kinetochore microtubles
- Kntc2:
-
kinetochore-associated protein 2
- MOI:
-
multiplicity of infection (encapsidated vector genome particles per cell)
- rAAV:
-
recombinant adeno-associated virus
- RNAi:
-
RNA interference
- Sf:
-
Spodoptera frugiperda
- shRNA:
-
short hairpin RNA
- siRNA:
-
small interfering RNA
References
Cheng JC, Moore TB, Sakamoto KM . RNA interference and human disease. Mol Genet Metab 2003; 80: 121–128.
Fuchs U, mm-Welk C, Borkhardt A . Silencing of disease-related genes by small interfering RNAs. Curr Mol Med 2004; 4: 507–517.
Heidenreich O . Oncogene suppression by small interfering RNAs. Curr Pharm Biotechnol 2004; 5: 349–354.
it-Si-Ali S, Guasconi V, Harel-Bellan A . RNA interference and its possible use in cancer therapy. Bull Cancer 2004; 91: 15–18.
Poliseno L, Mercatanti A, Citti L, Rainaldi G . RNA-based drugs: from RNA interference to short interfering RNAs. Curr Pharm Biotechnol 2004; 5: 361–368.
Scherer L, Rossi JJ . Recent applications of RNAi in mammalian systems. Curr Pharm Biotechnol 2004; 5: 355–360.
Lee NS, Dohjima T, Bauer G, Li H, Li MJ, Ehsani A et al. Expression of small interfering RNAs targeted against HIV-1 rev transcripts in human cells. Nat Biotechnol 2002; 20: 500–505.
Miyagishi M, Taira K . U6 promoter-driven siRNAs with four uridine 3′ overhangs efficiently suppress targeted gene expression in mammalian cells. Nat Biotechnol 2002; 20: 497–500.
Sui G, Soohoo C, Affar eB, Gay F, Shi Y, Forrester WC et al. A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci USA 2002; 99: 5515–5520.
Mailand N, Lukas C, Kaiser BK, Jackson PK, Bartek J, Lukas J . Deregulated human Cdc14A phosphatase disrupts centrosome separation and chromosome segregation. Nat Cell Biol 2002; 4: 317–322.
Paddison PJ, Caudy AA, Hannon GJ . Stable suppression of gene expression by RNAi in mammalian cells. Proc Natl Acad Sci USA 2002; 99: 1443–1448.
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T . Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature 2001; 411: 494–498.
Yu JY, DeRuiter SL, Turner DL . RNA interference by expression of short-interfering RNAs and hairpin RNAs in mammalian cells. Proc Natl Acad Sci USA 2002; 99: 6047–6052.
Brummelkamp TR, Bernards R, Agami R . A system for stable expression of short interfering RNAs in mammalian cells. Science 2002; 296: 550–553.
Harper SQ, Staber PD, He X, Eliason SL, Martins IH, Mao Q et al. RNA interference improves motor and neuropathological abnormalities in a Huntington's disease mouse model. Proc Natl Acad Sci USA 2005; 102: 5820–5825.
Hemann MT, Fridman JS, Zilfou JT, Hernando E, Paddison PJ, Cordon-Cardo C et al. An epi-allelic series of p53 hypomorphs created by stable RNAi produces distinct tumor phenotypes in vivo. Nat Genet 2003; 33: 396–400.
Tomar RS, Matta H, Chaudhary PM . Use of adeno-associated viral vector for delivery of small interfering RNA. Oncogene 2003; 22: 5712–5715.
Xia H, Mao Q, Eliason SL, Harper SQ, Martins IH, Orr HT et al. RNAi suppresses polyglutamine-induced neurodegeneration in a model of spinocerebellar ataxia. Nat Med 2004; 10: 816–820.
Ciferri C, De LJ, Monzani S, Ferrari KJ, Ristic D, Wyman C et al. Architecture of the human ndc80-hec1 complex, a critical constituent of the outer kinetochore. J Biol Chem 2005; 280: 29088–29095.
DeLuca JG, Dong Y, Hergert P, Strauss J, Hickey JM, Salmon ED et al. Hec1 and nuf2 are core components of the kinetochore outer plate essential for organizing microtubule attachment sites. Mol Biol Cell 2005; 16: 519–531.
DeLuca JG, Howell BJ, Canman JC, Hickey JM, Fang G, Salmon ED . Nuf2 and Hec1 are required for retention of the checkpoint proteins Mad1 and Mad2 to kinetochores. Curr Biol 2003; 13: 2103–2109.
Kline-Smith SL, Sandall S, Desai A . Kinetochore-spindle microtubule interactions during mitosis. Curr Opin Cell Biol 2005; 17: 35–46.
McCleland ML, Kallio MJ, Barrett-Wilt GA, Kestner CA, Shabanowitz J, Hunt DF et al. The vertebrate Ndc80 complex contains Spc24 and Spc25 homologs, which are required to establish and maintain kinetochore-microtubule attachment. Curr Biol 2004; 14: 131–137.
Stucke VM, Baumann C, Nigg EA . Kinetochore localization and microtubule interaction of the human spindle checkpoint kinase Mps1. Chromosoma 2004; 113: 1–15.
Emanuele MJ, McCleland ML, Satinover DL, Stukenberg PT . Measuring the stoichiometry and physical interactions between components elucidates the architecture of the vertebrate kinetochore. Mol Biol Cell 2005; 16: 4882–4892.
Bharadwaj R, Qi W, Yu H . Identification of two novel components of the human NDC80 kinetochore complex. J Biol Chem 2004; 279: 13076–13085.
Chen Y, Riley DJ, Zheng L, Chen PL, Lee WH . Phosphorylation of the mitotic regulator protein Hec1 by Nek2 kinase is essential for faithful chromosome segregation. J Biol Chem 2002; 277: 49408–49416.
Gurzov EN, Izquierdo M . RNA interference against Hec1 inhibits tumor growth in vivo. Gene Ther 2006; 13: 1–7.
McCarty DM, Young Jr SM, Samulski RJ . Integration of adeno-associated virus (AAV) and recombinant AAV vectors. Annu Rev Genet 2004; 38: 819–845.
Summerford C, Samulski RJ . Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J Virol 1998; 72: 1438–1445.
Summerford C, Bartlett JS, Samulski RJ . AlphaVbeta5 integrin: a co-receptor for adeno-associated virus type 2 infection. Nat Med 1999; 5: 78–82.
Kaludov N, Brown KE, Walters RW, Zabner J, Chiorini JA . Adeno-associated virus serotype 4 (AAV4) and AAV5 both require sialic acid binding for hemagglutination and efficient transduction but differ in sialic acid linkage specificity. J Virol 2001; 75: 6884–6893.
Akache B, Grimm D, Pandey K, Yant SR, Xu H, Kay MA . The 37/67-kilodalton laminin receptor is a receptor for adeno-associated virus serotypes 8, 2, 3, and 9. J Virol 2006; 80: 9831–9836.
Hacker UT, Wingenfeld L, Kofler DM, Schuhmann NK, Lutz S, Herold T et al. Adeno-associated virus serotypes 1 to 5 mediated tumor cell directed gene transfer and improvement of transduction efficiency. J Gene Med 2005; 7: 1429–1438.
Passini MA, Watson DJ, Vite CH, Landsburg DJ, Feigenbaum AL, Wolfe JH . Intraventricular brain injection of adeno-associated virus type 1 (AAV1) in neonatal mice results in complementary patterns of neuronal transduction to AAV2 and total long-term correction of storage lesions in the brains of beta-glucuronidase-deficient mice. J Virol 2003; 77: 7034–7040.
Reagan-Shaw S, Ahmad N . Silencing of polo-like kinase (Plk) 1 via siRNA causes induction of apoptosis and impairment of mitosis machinery in human prostate cancer cells: implications for the treatment of prostate cancer. FASEB J 2005; 19: 611–613.
Liu X, Erikson RL . Polo-like kinase (Plk)1 depletion induces apoptosis in cancer cells. Proc Natl Acad Sci USA 2003; 100: 5789–5794.
Ning S, Fuessel S, Kotzsch M, Kraemer K, Kappler M, Schmidt U et al. siRNA-mediated down-regulation of survivin inhibits bladder cancer cell growth. Int J Oncol 2004; 25: 1065–1071.
Guan HT, Xue XH, Dai ZJ, Wang XJ, Li A, Qin ZY . Down-regulation of survivin expression by small interfering RNA induces pancreatic cancer cell apoptosis and enhances its radiosensitivity. World J Gastroenterol 2006; 12: 2901–2907.
Mirza A, Basso A, Black S, Malkowski M, Kwee L, Pachter JA et al. RNA interference targeting of A1 receptor-overexpressing breast carcinoma cells leads to diminished rates of cell proliferation and induction of apoptosis. Cancer Biol Ther 2005; 4: 1355–1360.
Miyagishi M, Sumimoto H, Miyoshi H, Kawakami Y, Taira K . Optimization of an siRNA-expression system with an improved hairpin and its significant suppressive effects in mammalian cells. J Gene Med 2004; 6: 715–723.
Urabe M, Ding C, Kotin RM . Insect cells as a factory to produce adeno-associated virus type 2 vectors. Hum Gene Ther 2002; 13: 1935–1943.
Li L, Yang L, Kotin RM . The DNA minor groove binding agents Hoechst 33258 and 33342 enhance recombinant adeno-associated virus (rAAV) transgene expression. J Gene Med 2005; 7: 420–431.
Acknowledgements
We thank Scott Q Harper in Beverly Davidson's Laboratory at the University of Iowa and Wenqing Li at LMI of NCI, NIH for advise on shRNA design; Tracy Laabs in Herbert Geller's laboratory at NHLBI, NIH for providing primary CGNS cells; and Shigui Zhu at NHGRI, NIH for DNA sequencing. We acknowledge the professional skills and advice of NHLBI core facility members Philip McCoy Jr and Ann Williams at Flow Cytometry core; Fillipina Giacometti at Pathology Core; Christian A Combs and Daniela Malide at Light Microscopy Core regarding FACS-, pathology-, and microscopy-related experiments performed in this paper. We appreciate the help from Andres Mendez in our laboratory.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is a US Government work.
Rights and permissions
About this article
Cite this article
Li, L., Yang, L., Scudiero, D. et al. Development of recombinant adeno-associated virus vectors carrying small interfering RNA (shHec1)-mediated depletion of kinetochore Hec1 protein in tumor cells. Gene Ther 14, 814–827 (2007). https://doi.org/10.1038/sj.gt.3302933
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.gt.3302933
Keywords
This article is cited by
-
N-terminus-modified Hec1 suppresses tumour growth by interfering with kinetochore–microtubule dynamics
Oncogene (2015)
-
Novel small molecules disrupting Hec1/Nek2 interaction ablate tumor progression by triggering Nek2 degradation through a death-trap mechanism
Oncogene (2015)
-
An RNAi-based screen reveals PLK1, CDK1 and NDC80 as potential therapeutic targets in malignant pleural mesothelioma
British Journal of Cancer (2014)
-
Mitosis as an anti-cancer drug target
Chromosoma (2013)
-
Selective gene silencing by viral delivery of short hairpin RNA
Virology Journal (2010)